How brain diseases affect the lower urinary tract function?

This article reviewed brain mechanism of the lower urinary tract (LUT). Among autonomic nervous systems, LUT is unique in terms of afferent pathophysiology; bladder sensation is perceived soon after the storage phase and throughout the voiding phase. Within the brain, this is measured in experimental animals by the firing of single neurons and in humans by evoked potentials/functional neuroimaging. The evidence indicates that sphincter information goes up to the precentral motor cortex and other brain areas, and bladder information goes up to the insular cortex (IC)/anterior cingulate (ACG) and further to the prefrontal cortex (PFC). Another LUT-specific phenomenon is efferent pathophysiology: detrusor overactivity (exaggerated micturition reflex) occurs in brain diseases such as stroke (focal disease) and dementia with Lewy bodies (diffuse diseases, may overlap with each other). With the turning off and on of the brain-switch of mictu-rition (at the periaqueductal gray [PAG]), there is a bladder-inhibitory PFC-IC/ACG-hypothalamus-PAG pathway, with interconnections via the PFC with a PFC-nigrostriatal D1 dopaminergic pathway and a PFC-cerebellar pathway. Brain diseases that affect these areas may cause a loss of the brain's inhibition of the micturition reflex, leading to detrusor overactivity. This has a significant clinical impact on patients and requires appropriate management.
INTRODUCTION
Brain control of the lower urinary tract
Peripheral innervation of the LUT
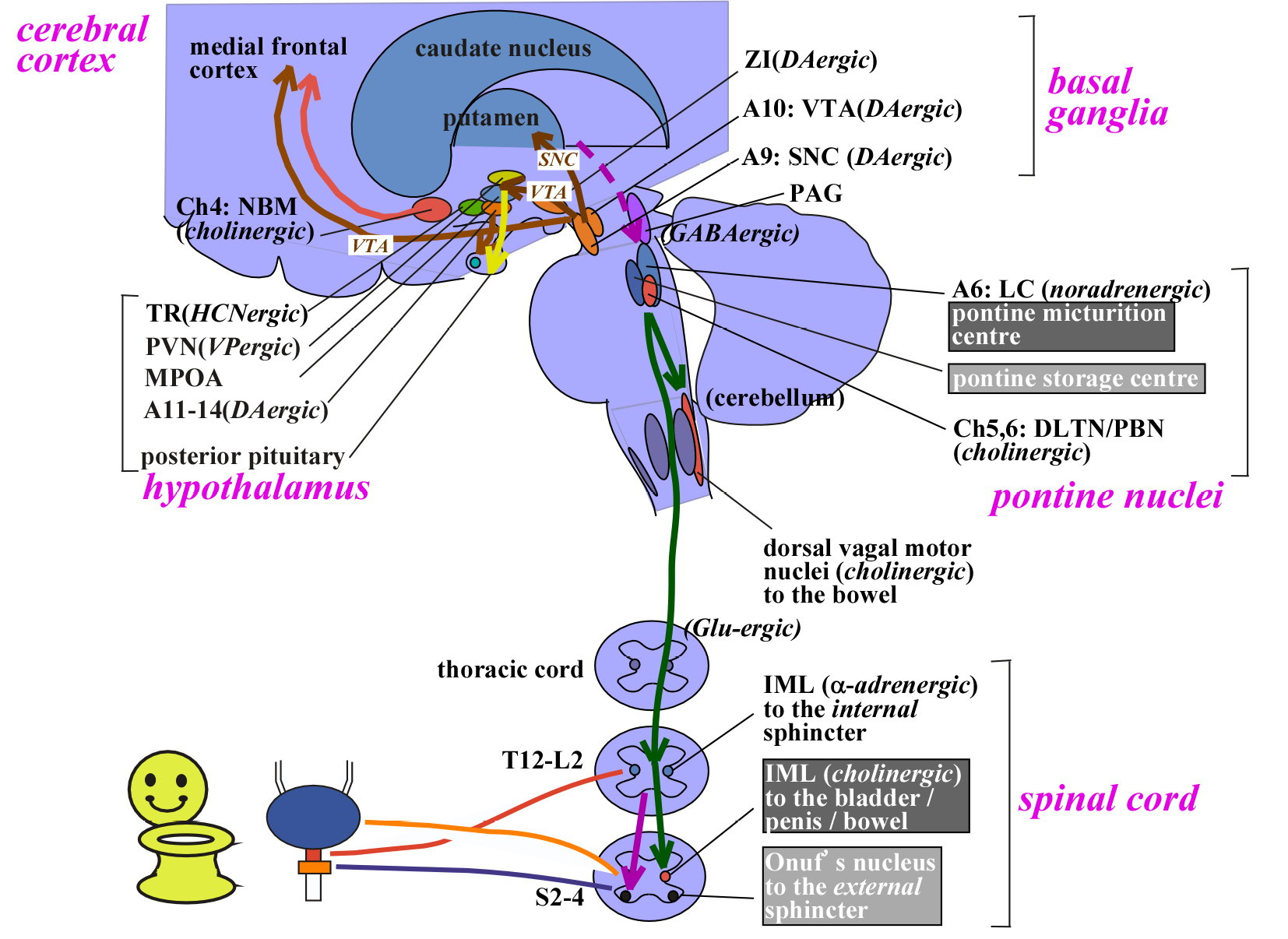
Notes: A, adrenergic/noradrenergic; DLTN, dorsolateral tegmental nucleus; GABA, gamma-aminobutyric acid; IML, intermediolateral cell column; L, lumbar; MPOA, medial preoptic area; PBN, parabrachial nucleus; PVN, paraventricular nucleus; S, sacral; SNC, substantia nigra pars compacta; T, thoracic; VTA, ventral tegmental area; ZI, zona incerta.
Role of the brain for storage and voiding function of the LUT
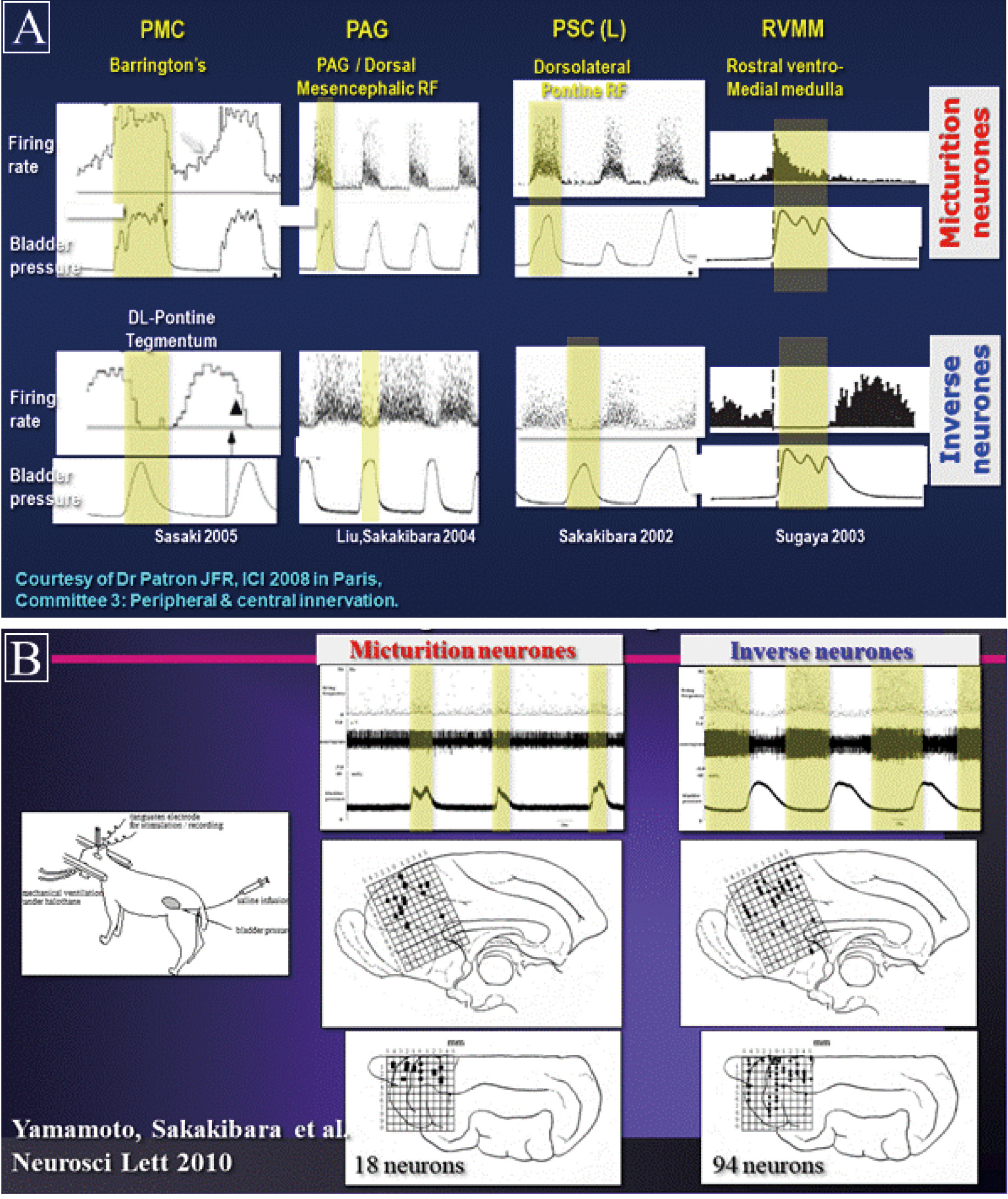
Neuronal recording studies and neurotransmitters
Neuronal stimulation studies
Neuroimaging studies relevant to LUT function
Notes: Figure B was cited from Reference 24.
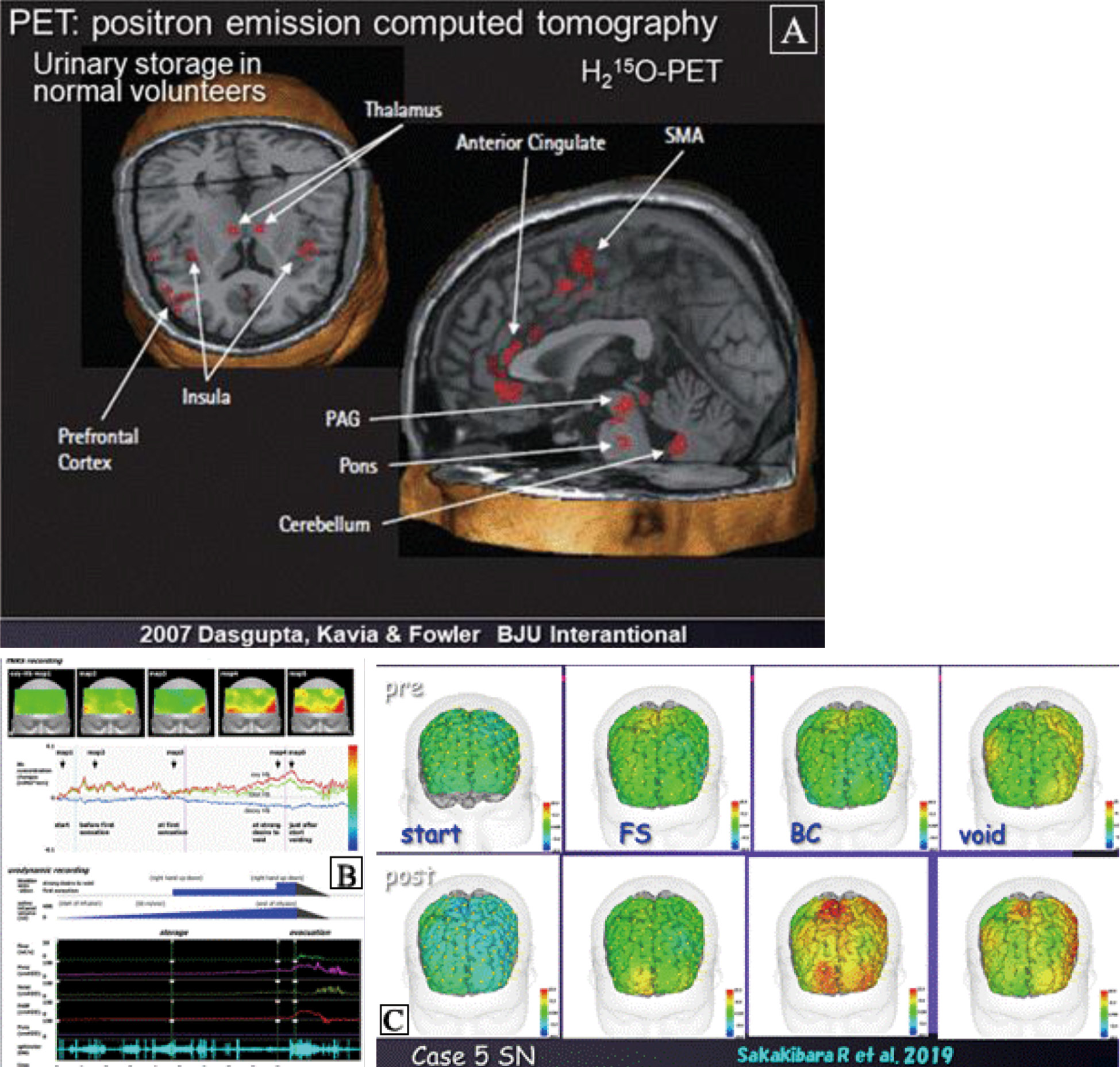
Notes: Figure 3A and 3B were cited from Reference 38.
Electrosensation studies
Brain area relevant to the LUTS: cortical structures
Brain area relevant to the LUTS: basal ganglia, cerebellum and other deep structures
Brain diseases affecting the lower urinary tract function
Focal, single brain diseases (stroke)
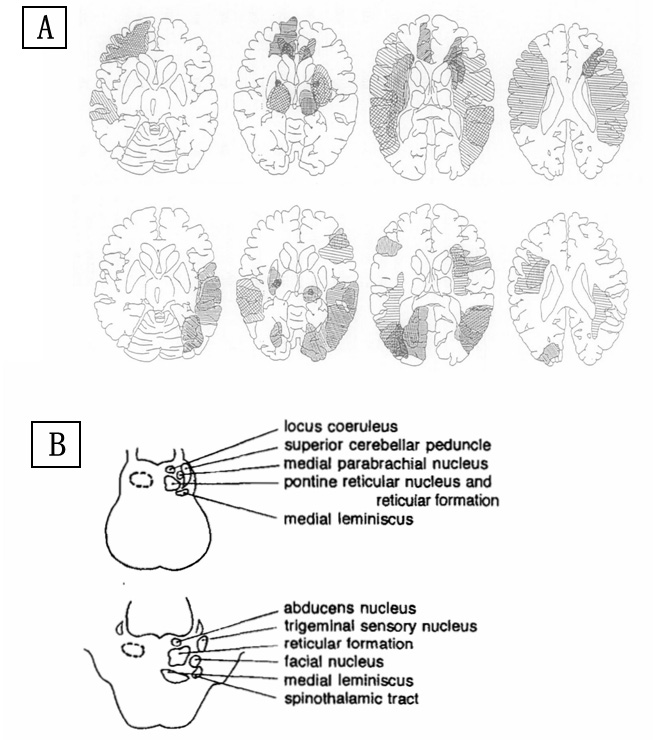
Notes: Figure 4A was cited from Reference 107; Figure 4B was cited from Reference 109.
Diffuse brain diseases in older individuals (AD, WMD, and DLB [Parkinson's disease plus])
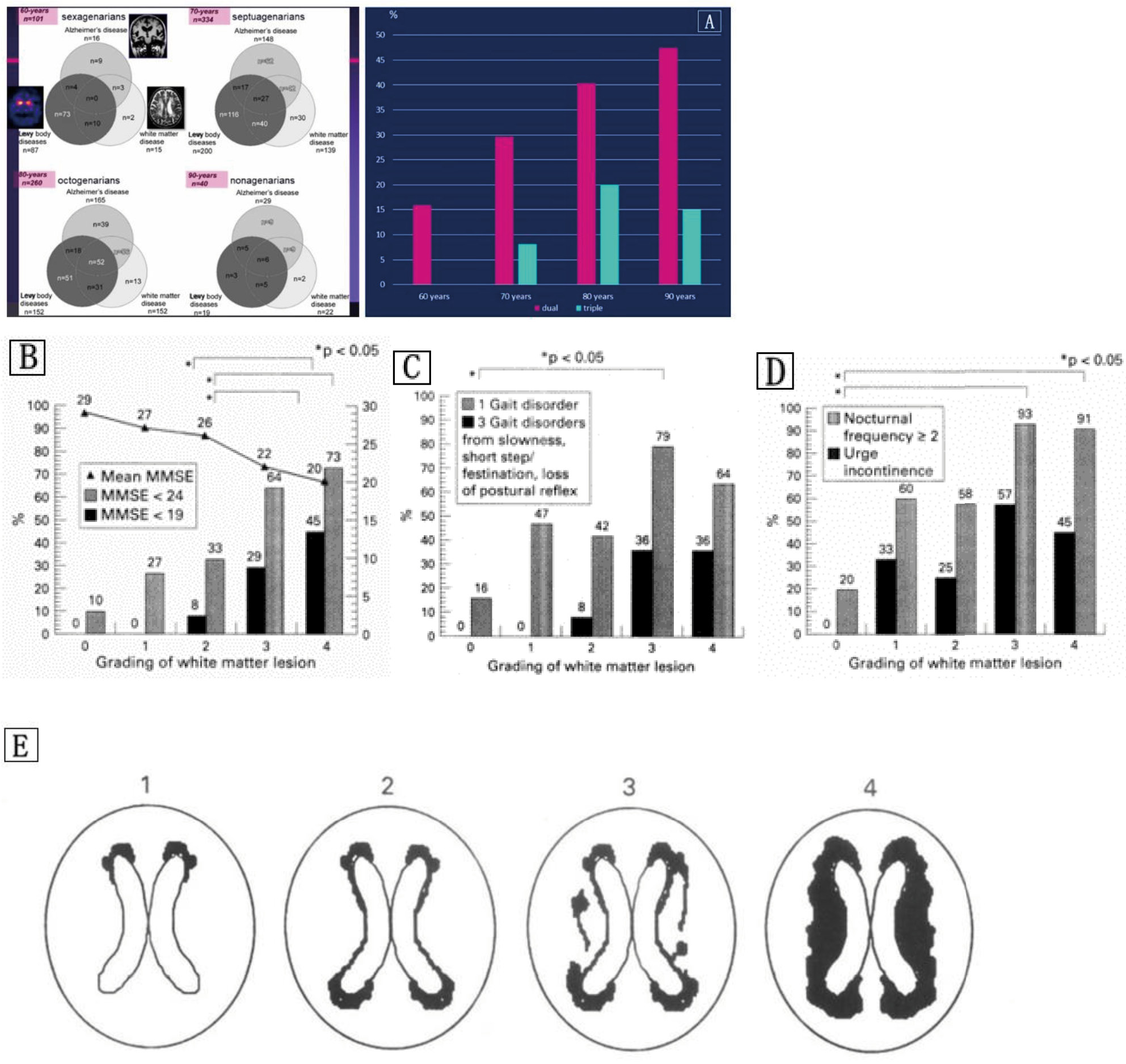
Notes: Figure B‒D were cited from Reference 115.
CONCLUSION
- de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015 Jan;5(1):327–96. PMID: 25589273
- Andersson KE, Birder L, Chermansky C, Chess-Williams R, Fry C. Are there relevant animal models to set research priorities in LUTD? ICI-RS 2019. Neurourol Urodyn. 2020 Jul;39(Suppl 3 Suppl 3):S9–15. https://doi.org/10.1002/nau.24259 PMID: 32662562
- Drake MJ, Fowler CJ, Griffiths D, Mayer E, Paton JF, Birder L. Neural control of the lower urinary and gastrointestinal tracts: supraspinal CNS mechanisms. Neurourol Urodyn. 2010;29(1):119–27. https://doi.org/10.1002/nau.20841 PMID: 20025025
- Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29(1):49–55. https://doi.org/10.1002/nau.20740 PMID: 19412958
- Griffiths DJ, Fowler CJ. The micturition switch and its forebrain influences. Acta Physiol (Oxf). 2013 Jan;207(1):93–109. https://doi.org/10.1111/apha.12019 PMID: 23164237
- Sakakibara R, Fowler CJ. Chapter 9: Brain disease. In: Fowler CJ, editor. Seminars in Clinical Neurology (by World Federation of Neurology). Neurologic bladder, bowel, and sexual function. Boston: Elsevier; 2001. pp. 229–43.
- Blok BF, Holstege G. The central control of micturition and continence: implications for urology. BJU Int. 1999 Mar;83(S2 Suppl 2):1–6. https://doi.org/10.1046/j.1464-410X.83.s2.2.x PMID: 10210596
- Sakakibara R, Nakazawa K, Shiba K, Nakajima Y, Uchiyama T, Yoshiyama M, et al. Firing patterns of micturition-related neurons in the pontine storage centre in cats. Auton Neurosci. 2002 Jul;99(1):24–30. https://doi.org/10.1016/S1566-0702(02)00055-3 PMID: 12171253
- Noto H, Roppolo JR, Steers WD, de Groat WC. Electrophysiological analysis of the ascending and descending components of the micturition reflex pathway in the rat. Brain Res. 1991 May;549(1):95–105. https://doi.org/10.1016/0006-8993(91)90604-T PMID: 1893257
- Kruse MN, Mallory BS, Noto H, Roppolo JR, de Groat WC. Modulation of the spinobulbospinal micturition reflex pathway in cats. Am J Physiol. 1992 Mar;262(3 Pt 2):R478–84. PMID: 1558219
- Liu Z, Sakakibara R, Nakazawa K, Uchiyama T, Yamamoto T, Ito T, et al. Micturition-related neuronal firing in the periaqueductal gray area in cats. Neuroscience. 2004;126(4):1075–82. https://doi.org/10.1016/j.neuroscience.2004.04.033 PMID: 15207340
- Zare A, Schipper S, Stein W, Temel Y, van Koeveringe GA, Jahanshahi A. Electrophysiological responses of the ventrolateral periaqueductal gray matter neurons towards peripheral bladder stimulation. Brain Res Bull. 2018 Sep;142:116–21. https://doi.org/10.1016/j.brainresbull.2018.07.009 PMID: 30016723
- Sugaya K, Ogawa Y, Hatano T, Nishijima S, Matsuyama K, Mori S. Ascending and descending brainstem neuronal activity during cystometry in decerebrate cats. Neurourol Urodyn. 2003;22(4):343–50. https://doi.org/10.1002/nau.10115 PMID: 12808711
- Ito T, Sakakibara R, Nakazawa K, Uchiyama T, Yamamoto T, Liu Z, et al. Effects of electrical stimulation of the raphe area on the micturition reflex in cats. Neuroscience. 2006 Nov;142(4):1273–80. https://doi.org/10.1016/j.neuroscience.2006.06.044 PMID: 16996219
- Rocha I, Silva-Carvalho L, Spyer KM. Effect of stimulation of anterior hypothalamic area on urinary bladder function of the anesthetized rat. Clin Auton Res. 2004 Aug;14(4):264–9. https://doi.org/10.1007/s10286-004-0212-0 PMID: 15316845
- Verstegen AM, Klymko N, Zhu L, Mathai JC, Kobayashi R, Venner A, et al. Non-Crh glutamatergic neurons in Barrington’s nucleus control micturition via glutamatergic afferents from the midbrain and hypothalamus. Curr Biol. 2019 Sep;29(17):2775–2789.e7. https://doi.org/10.1016/j.cub.2019.07.009 PMID: 31422881
- Sakakibara R, Nakazawa K, Uchiyama T, Yoshiyama M, Yamanishi T, Hattori T. Micturition-related electrophysiological properties in the substantia nigra pars compacta and the ventral tegmental area in cats. Auton Neurosci. 2002 Nov;102(1-2):30–8. https://doi.org/10.1016/S1566-0702(02)00180-7 PMID: 12492133
- Yamamoto T, Sakakibara R, Hashimoto K, Nakazawa K, Uchiyama T, Liu Z, et al. Striatal dopamine level increases in the urinary storage phase in cats: an in vivo microdialysis study. Neuroscience. 2005;135(1):299–303. https://doi.org/10.1016/j.neuroscience.2005.06.007 PMID: 16111828
- Yamamoto T, Sakakibara R, Nakazawa K, Uchiyama T, Shimizu E, Hattori T. Effects of electrical stimulation of the striatum on bladder activity in cats. Neurourol Urodyn. 2009;28(6):549–54. https://doi.org/10.1002/nau.20682 PMID: 19214990
- Sakakibara R, Nakazawa K, Uchiyama T, Yoshiyama M, Yamanishi T, Hattori T. Effects of subthalamic nucleus stimulation on the micturation reflex in cats. Neuroscience. 2003;120(3):871–5. https://doi.org/10.1016/S0306-4522(03)00319-1 PMID: 12895527
- Yamamoto T, Uchiyama T, Sakakibara R, Taniguchi J, Kuwabara S. The subthalamic activity and striatal monoamine are modulated by subthalamic stimulation. Neuroscience. 2014 Feb;259:43–52. https://doi.org/10.1016/j.neuroscience.2013.11.034 PMID: 24291727
- Yamamoto T, Sakakibara R, Uchiyama T, Kuwabara S. Subthalamic stimulation inhibits bladder contraction by modulating the local field potential and catecholamine level of the medial prefrontal cortex. Front Neurosci. 2020 Sep;14:917. https://doi.org/10.3389/fnins.2020.00917 PMID: 33013304
- Koyama K. Effects of amygdaloid and olfactory tubercle stimulation on efferent activities of the vesical branch of the pelvic nerve and the urethral branch of the pudendal nerve in dogs. Urol Int. 1991;47 Suppl 1:23–30. https://doi.org/10.1159/000282244 PMID: 1659014
- Yamamoto T, Sakakibara R, Nakazawa K, Uchiyama T, Shimizu E, Hattori T, et al. Neuronal activities of forebrain structures with respect to bladder contraction in cats. Neurosci Lett. 2010 Mar;473(1):42–7. https://doi.org/10.1016/j.neulet.2010.02.015 PMID: 20153810
- Brüggemann J, Vahle-Hinz C, Kniffki KD. Representation of the urinary bladder in the lateral thalamus of the cat. J Neurophysiol. 1993 Aug;70(2):482–91. https://doi.org/10.1152/jn.1993.70.2.482 PMID: 8410150
- Robbins MT, Uzzell TW, Aly S, Ness TJ. Characterization of thalamic neuronal responses to urinary bladder distention, including the effect of acute spinal lesions in the rat. J Pain. 2006 Mar;7(3):218–24. https://doi.org/10.1016/j.jpain.2005.10.012 PMID: 16516828
- Sasaki M. Role of Barrington’s nucleus in micturition. J Comp Neurol. 2005 Dec;493(1):21–6. https://doi.org/10.1002/cne.20719 PMID: 16255005
- Sasaki M. Properties of Barrington’s neurones in cats: units that fire inversely with micturition contraction. Brain Res. 2005 Feb;1033(1):41–50. https://doi.org/10.1016/j.brainres.2004.11.016 PMID: 15680338
- Ito H, Sales AC, Fry CH, Kanai AJ, Drake MJ, Pickering AE. Probabilistic, spinally-gated control of bladder pressure and autonomous micturition by Barrington’s nucleus CRH neurons. eLife. 2020 Apr;9:e56605. https://doi.org/10.7554/eLife.56605 PMID: 32347794
- Keller JA, Chen J, Simpson S, Wang EH, Lilascharoen V, George O, et al. Voluntary urination control by brainstem neurons that relax the urethral sphincter. Nat Neurosci. 2018 Sep;21(9):1229–38. https://doi.org/10.1038/s41593-018-0204-3 PMID: 30104734
- Gjone R. Excitatory and inhibitory bladder responses to stimulation of ‘limbic’, diencephalic and mesencephalic structures in the cat. Acta Physiol Scand. 1966;66(1):91–102. https://doi.org/10.1111/j.1748-1716.1966.tb03171.x PMID: 5327579
- Nakagawa S. Onuf’s nucleus of the sacral cord in a South American monkey (Saimiri): its location and bilateral cortical input from area 4. Brain Res. 1980 Jun;191(2):337–44. https://doi.org/10.1016/0006-8993(80)91285-8 PMID: 6769542
- Sem-Jacobson CW. Depth electrographic stimulation of the human brain and behavior. Springfield: Thomas; 1968.
- Patra S, Valls L, Heredia G, Burdette D, Elisevich K. Human left anterior cingulate stimulation elicits a reproducible micturition response. Stereotact Funct Neurosurg. 2019;97(4):278–81. https://doi.org/10.1159/000503886 PMID: 31751999
- Brusa L, Finazzi Agrò E, Petta F, Sciobica F, Torriero S, Lo Gerfo E, et al. Effects of inhibitory rTMS on bladder function in Parkinson’s disease patients. Mov Disord. 2009 Feb;24(3):445–8. https://doi.org/10.1002/mds.22434 PMID: 19133657
- Fukuyama H, Matsuzaki S, Ouchi Y, Yamauchi H, Nagahama Y, Kimura J, et al. Neural control of micturition in man examined with single photon emission computed tomography using 99mTc-HMPAO. Neuroreport. 1996 Nov;7(18):3009–12. https://doi.org/10.1097/00001756-199611250-00042 PMID: 9116229
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997 Jan;120(Pt 1):111–21. https://doi.org/10.1093/brain/120.1.111 PMID: 9055802
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001 Feb;124(Pt 2):369–77. https://doi.org/10.1093/brain/124.2.369 PMID: 11157564
- DasGupta R, Kavia RB, Fowler CJ. Cerebral mechanisms and voiding function. BJU Int. 2007 Apr;99(4):731–4. https://doi.org/10.1111/j.1464-410X.2007.06749.x PMID: 17378838
- Jarrahi B, Mantini D, Balsters JH, Michels L, Kessler TM, Mehnert U, et al. Differential functional brain network connectivity during visceral interoception as revealed by independent component analysis of fMRI TIME-series. Hum Brain Mapp. 2015 Nov;36(11):4438–68. https://doi.org/10.1002/hbm.22929 PMID: 26249369
- Coolen RL, Groenendijk IM, Blok BF. Recent advances in neuroimaging of bladder, bowel and sexual function. Curr Opin Urol. 2020 Jul;30(4):480–5. https://doi.org/10.1097/MOU.0000000000000772 PMID: 32427628
- Jarrahi B, Kollias S. Effects of visceral interoception on topological properties of the brain – A graph theory analysis of resting state fMRI. Annu Int Conf IEEE Eng Med Biol Soc. 2020 Jul;2020:1116–9. https://doi.org/10.1109/EMBC44109.2020.9175465 PMID: 33018182
- Pang D, Gao Y, Liao L. Responses of functional brain networks to bladder control in healthy adults: a study using regional homogeneity combined with independent component analysis methods. Int Urol Nephrol. 2021 May;53(5):883–91. https://doi.org/10.1007/s11255-020-02742-1 PMID: 33523398
- Tsunoyama K, Sakakibara R, Takahashi O, Sugiyama M, Uchiyama T, Tateno F, et al. How the bladder senses? A five-grade measure. Low Urin Tract Symptoms. 2013 Jan;5(1):17–22. https://doi.org/10.1111/j.1757-5672.2012.00156.x PMID: 26663243
- Sakakibara R, Tateno F, Sugiyama M, Takahashi O, Nishimura H, Kishi M, et al. How to measure bladder sensation in peripheral nerve diseases? Curr Bladder Dysfunct Rep. 2017;12(4):241–5. https://doi.org/10.1007/s11884-017-0451-2.
- Tateno F, Sakakibara R, Aiba Y, Takahashi O, Shimizu A, Oki T. Increased bladder sensation without detrusor overactivity revisited: use of a five-grade sensory measure. Low Urin Tract Symptoms. 2020 May;12(2):162–6. https://doi.org/10.1111/luts.12298 PMID: 31833660
- Sakakibara R, Tsunoyama K, Takahashi O, Sugiyama M, Kishi M, Ogawa E, et al. Real-time measurement of oxyhemoglobin concentration changes in the frontal micturition area: an fNIRS study. Neurourol Urodyn. 2010 Jun;29(5):757–64. https://doi.org/10.1002/nau.20815 PMID: 20583001
- Sakakibara R, Tateno F, Yano M, Takahashi O, Sugiyama M, Ogata T, et al. Imidafenacin on bladder and cognitive function in neurologic OAB patients. Clin Auton Res. 2013 Aug;23(4):189–95. https://doi.org/10.1007/s10286-013-0200-3 PMID: 23820664
- Sakakibara R, Tateno F, Yano M, Takahashi O, Aiba Y, Yamamoto T. Fesoterodine normalizes the brain function in overactive bladder patients due to central nervous system lesion: A real-time measure of oxyhemoglobin concentration changes during urodynamics. Int J Urol. 2019 Oct;26(10):1014–5. https://doi.org/10.1111/iju.14072 PMID: 31309606
- Wyndaele JJ. Studies on sensory threshold of different parts of the lower urinary tract measured electrically. Eur Urol. 1991;19(2):121–4. https://doi.org/10.1159/000473599 PMID: 2022214
- Haldeman S, Bradley WE, Bhatia NN, Johnson BK. Pudendal evoked responses. Arch Neurol. 1982 May;39(5):280–3. https://doi.org/10.1001/archneur.1982.00510170022006 PMID: 7073545
- McGee MJ, Swan BD, Danziger ZC, Amundsen CL, Grill WM. Multiple reflex pathways contribute to bladder activation by intraurethral stimulation in persons with spinal cord injury. Urology. 2017 Nov;109:210–5. https://doi.org/10.1016/j.urology.2017.07.041 PMID: 28801220
- Markland C, Bradley W, Chou S, Merrill D, Westgate H. Sacral nerve stimulation. A diagnostic test of bladder innervation. Br J Urol. 1971 Aug;43(4):453–9. https://doi.org/10.1111/j.1464-410X.1971.tb12068.x PMID: 4937823
- Averbeck MA, Moreno-Palacios J, Aparicio A. Is there a role for sacral neuromodulation in patients with neurogenic lower urinary tract dysfunction? Int Braz J Urol. 2020;46(6):891–901. https://doi.org/10.1590/s1677-5538.ibju.2020.99.10 PMID: 32758301
- Assmann R, Douven P, Kleijnen J, van Koeveringe GA, Joosten EA, Melenhorst J, et al. Stimulation parameters for sacral neuromodulation on lower urinary tract and bowel dysfunction-related clinical outcome: A systematic review. Neuromodulation. 2020 Dec;23(8):1082–93. https://doi.org/10.1111/ner.13255 PMID: 32830414
- Abouassaly R, Liu G, Yamada Y, Ukimura O, Daneshgari F. Efficacy of a novel device for assessment of autonomic sensory function in the rat bladder. J Urol. 2008 Mar;179(3):1167–72. https://doi.org/10.1016/j.juro.2007.10.027 PMID: 18206176
- Bicer F, Kim JY, Horowitz A, Daneshgari F, Liu G. Assessment of bladder sensation in mice with a novel device. Urology. 2014 Aug;84(2):490.e1–6. https://doi.org/10.1016/j.urology.2014.04.024 PMID: 24958485
- De Wachter S, Van Meel TD, Wyndaele JJ. Study of the afferent nervous system and its evaluation in women with impaired detrusor contractility treated with bethanechol. Urology. 2003 Jul;62(1):54–8. https://doi.org/10.1016/S0090-4295(03)00246-2 PMID: 12837422
- Yokoyama T, Nozaki K, Fujita O, Nose H, Inoue M, Kumon H. Role of C afferent fibers and monitoring of intravesical resiniferatoxin therapy for patients with idiopathic detrusor overactivity. J Urol. 2004 Aug;172(2):596–600. https://doi.org/10.1097/01.ju.0000132769.71014.b5 PMID: 15247740
- Kenton K, Simmons J, FitzGerald MP, Lowenstein L, Brubaker L. Urethral and bladder current perception thresholds: normative data in women. J Urol. 2007 Jul;178(1):189–92. https://doi.org/10.1016/j.juro.2007.03.032 PMID: 17499783
- Kenton K, Lowenstein L, Brubaker L. Tolterodine causes measurable restoration of urethral sensation in women with urge urinary incontinence. Neurourol Urodyn. 2010 Apr;29(4):555–7. https://doi.org/10.1002/nau.20804 PMID: 19771598
- Van Meel TD, De Wachter S, Wyndaele JJ. The effect of intravesical oxybutynin on the ice water test and on electrical perception thresholds in patients with neurogenic detrusor overactivity. Neurourol Urodyn. 2010 Mar;29(3):391–4. https://doi.org/10.1002/nau.20785 PMID: 19787712
- Fujihara A, Ukimura O, Iwata T, Miki T. Neuroselective measure of the current perception threshold of A-delta and C-fiber afferents in the lower urinary tract. Int J Urol. 2011 May;18(5):341–9. https://doi.org/10.1111/j.1442-2042.2011.02749.x PMID: 21443728
- Vijaya G, Digesu GA, Derpapas A, Hendricken C, Fernando R, Khullar V. Antimuscarinic effects on current perception threshold: a prospective placebo control study. Neurourol Urodyn. 2012 Jan;31(1):75–9. https://doi.org/10.1002/nau.21194 PMID: 22038939
- van der Lely S, Kessler TM, Mehnert U, Liechti MD. Scalp topography of lower urinary tract sensory evoked potentials. Brain Topogr. 2020 Nov;33(6):693–709. https://doi.org/10.1007/s10548-020-00796-z PMID: 33067692
- Matsushita M, Nakasato N, Nakagawa H, Kanno A, Kaiho Y, Arai Y. Primary somatosensory evoked magnetic fields elicited by sacral surface electrical stimulation. Neurosci Lett. 2008 Jan;431(1):77–80. https://doi.org/10.1016/j.neulet.2007.11.025 PMID: 18162313
- Bradley WE. Cerebro-cortical innervation of the urinary bladder. Tohoku J Exp Med. 1980 May;131(1):7–13. https://doi.org/10.1620/tjem.131.7 PMID: 6250250
- Haldeman S, Bradley WE, Bhatia NN, Johnson BK. Pudendal evoked responses. Arch Neurol. 1982 May;39(5):280–3. https://doi.org/10.1001/archneur.1982.00510170022006 PMID: 7073545
- Sakakibara R. Lower urinary tract dysfunction in patients with brain lesions. Handb Clin Neurol. 2015;130:269–87. https://doi.org/10.1016/B978-0-444-63247-0.00015-8 PMID: 26003249
- Michels L, Blok BF, Gregorini F, Kurz M, Schurch B, Kessler TM, et al. Supraspinal control of urine storage and micturition in men—an fMRI study. Cereb Cortex. 2015 Oct;25(10):3369–80. https://doi.org/10.1093/cercor/bhu140 PMID: 24969474
- Zhao L, Liao L, Gao Y. Brain functional connectivity during storage based on resting state functional magnetic resonance imaging with synchronous urodynamic testing in healthy volunteers. Brain Imaging Behav. 2020;•••: https://doi.org/10.1007/s11682-020-00362-y PMID: 32725470
- Groenendijk IM, Mehnert U, Groen J, Clarkson BD, Scheepe JR, Blok BF. A systematic review and activation likelihood estimation meta-analysis of the central innervation of the lower urinary tract: pelvic floor motor control and micturition. PLoS One. 2021 Feb;16(2):e0246042. https://doi.org/10.1371/journal.pone.0246042 PMID: 33534812
- Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage. 2008 Feb;39(4):1647–53. https://doi.org/10.1016/j.neuroimage.2007.10.059 PMID: 18089297
- Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27(6):466–74. https://doi.org/10.1002/nau.20549 PMID: 18092336
- Tai C, Wang J, Jin T, Wang P, Kim SG, Roppolo JR, et al. Brain switch for reflex micturition control detected by FMRI in rats. J Neurophysiol. 2009 Nov;102(5):2719–30. https://doi.org/10.1152/jn.00700.2009 PMID: 19741099
- Yao J, Zhang Q, Liao X, Li Q, Liang S, Li X, et al. A corticopontine circuit for initiation of urination. Nat Neurosci. 2018 Nov;21(11):1541–50. https://doi.org/10.1038/s41593-018-0256-4 PMID: 30361547
- Haruta H, Sakakibara R, Ogata T, Panicker J, Fowler CJ, Tateno F, et al. Inhibitory control task is decreased in vascular incontinence patients. Clin Auton Res. 2013 Apr;23(2):85–9. https://doi.org/10.1007/s10286-013-0187-9 PMID: 23334165
- Haruta H, Sakakibara R, Ogata T, Tateno F, Kishi M, Aiba Y, et al. Frontal executive function and the bladder: A study of dementia with Lewy bodies. Neurol Clin Neurosci. 2018;7(1):22–5. https://doi.org/10.1111/ncn3.12237.
- Choi EY, Tanimura Y, Vage PR, Yates EH, Haber SN. Convergence of prefrontal and parietal anatomical projections in a connectional hub in the striatum. Neuroimage. 2017 Feb;146:821–32. https://doi.org/10.1016/j.neuroimage.2016.09.037 PMID: 27646127
- Shepherd GM, Griller S. Handbook of brain microcircuits. Oxford: Oxford University Press; 2010.ISBN: 978-0190636111. https://doi.org/10.1093/med/9780195389883.001.0001
- Hupalo S, Martin AJ, Green RK, Devilbiss DM, Berridge CW. Prefrontal corticotropin-releasing factor (CRF) neurons act locally to modulate frontostriatal cognition and circuit function. J Neurosci. 2019 Mar;39(11):2080–90. https://doi.org/10.1523/JNEUROSCI.2701-18.2019 PMID: 30651328
- Sha Z, Versace A, Edmiston EK, Fournier J, Graur S, Greenberg T, et al. Functional disruption in prefrontal-striatal network in obsessive-compulsive disorder. Psychiatry Res Neuroimaging. 2020 Jun;300:111081. https://doi.org/10.1016/j.pscychresns.2020.111081 PMID: 32344156
- Yamamoto T, Sakakibara R, Nakazawa K, Uchiyama T, Shimizu E, Hattori T. Effects of electrical stimulation of the striatum on bladder activity in cats. Neurourol Urodyn. 2009;28(6):549–54. https://doi.org/10.1002/nau.20682 PMID: 19214990
- Sakakibara R, Nakazawa K, Uchiyama T, Yoshiyama M, Yamanishi T, Hattori T. Effects of subthalamic nucleus stimulation on the micturation reflex in cats. Neuroscience. 2003;120(3):871–5. https://doi.org/10.1016/S0306-4522(03)00319-1 PMID: 12895527
- Herzog J, Weiss PH, Assmus A, Wefer B, Seif C, Braun PM, et al. Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain. 2008 Jan;131(Pt 1):132–45. PMID: 17977862
- Sakakibara R, Kishi M, Ogawa E, Tateno F, Uchiyama T, Yamamoto T, et al. Bladder, bowel, and sexual dysfunction in Parkinson’s disease. Parkinsons Dis. 2011;2011:924605. https://doi.org/10.4061/2011/924605 PMID: 21918729
- Sakakibara R, Tateno F, Yamamoto T, Uchiyama T, Yamanishi T. Urological dysfunction in synucleinopathies: epidemiology, pathophysiology and management. Clin Auton Res. 2018 Feb;28(1):83–101. https://doi.org/10.1007/s10286-017-0480-0 PMID: 29124503
- Terayama K, Sakakibara R, Ogawa A, Haruta H, Akiba T, Nagao T, et al. Weak detrusor contractility correlates with motor disorders in Parkinson’s disease. Mov Disord. 2012 Dec;27(14):1775–80. https://doi.org/10.1002/mds.25225 PMID: 23080035
- Benagiano V, Rizzi A, Lorusso L, Flace P, Saccia M, Cagiano R, et al. The functional anatomy of the cerebrocerebellar circuit: A review and new concepts. J Comp Neurol. 2018 Apr;526(5):769–89. https://doi.org/10.1002/cne.24361 PMID: 29238972
- Beuriat PA, Cohen-Zimerman S, Smith GN, Krueger F, Gordon B, Grafman J. A new insight on the role of the cerebellum for executive functions and emotion processing in adults. Front Neurol. 2020 Dec;11:593490. https://doi.org/10.3389/fneur.2020.593490 PMID: 33424746
- Huang TF, Yang CP, Yang SL. The role of the fastigial nucleus in bladder control. Exp Neurol. 1979 Dec;66(3):674–81. https://doi.org/10.1016/0014-4886(79)90212-7 PMID: 488245
- Huang TF, Yang CP, Yang SL. The role of the fastigial nucleus in bladder control. Exp Neurol. 1979 Dec;66(3):674–81. https://doi.org/10.1016/0014-4886(79)90212-7 PMID: 488245
- Tateno F, Sakakibara R, Sugiyama M, Kishi M, Ogawa E, Takahashi O, et al. Lower urinary tract function in spinocerebellar ataxia 6. Low Urin Tract Symptoms. 2012 Jan;4(1):41–4. https://doi.org/10.1111/j.1757-5672.2011.00111.x PMID: 26676458
- Tai C, Wang J, Jin T, Wang P, Kim SG, Roppolo JR, et al. Brain switch for reflex micturition control detected by FMRI in rats. J Neurophysiol. 2009 Nov;102(5):2719–30. https://doi.org/10.1152/jn.00700.2009 PMID: 19741099
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003 Dec;26(4):331–43. https://doi.org/10.1016/j.jchemneu.2003.10.002 PMID: 14729135
- de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology. 2002 May;59(5 Suppl 1):30–6. https://doi.org/10.1016/S0090-4295(01)01636-3 PMID: 12007520
- Chiba H, Kitta T, Ohmura Y, Higuchi M, Kon M, Nakamura M, et al. Serotonin in the rat prefrontal cortex controls the micturition reflex through 5-hydroxytryptamine 2A and 5-hydroxytryptamine 7 receptors. Int J Urol. 2020 Aug;27(8):684–9. https://doi.org/10.1111/iju.14267 PMID: 32533581
- Sakakibara R, Ito T, Yamamoto T, Uchiyama T, Yamanishi T, Kishi M, et al. Depression, anxiety and the bladder. Low Urin Tract Symptoms. 2013 Sep;5(3):109–20. https://doi.org/10.1111/luts.12018 PMID: 26663445
- Carandini T, Mancini M, Bogdan I, Rae CL, Barritt AW, Sethi A, et al. Disruption of brainstem monoaminergic fibre tracts in multiple sclerosis as a putative mechanism for cognitive fatigue: a fixel-based analysis. Neuroimage Clin. 2021;30:102587. https://doi.org/10.1016/j.nicl.2021.102587;Onlineaheadofprint. PMID: 33610097
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al.; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002 Jul;187(1):116–26. https://doi.org/10.1067/mob.2002.125704 PMID: 12114899
- Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Dementia and lower urinary dysfunction: with a reference to anticholinergic use in elderly population. Int J Urol. 2008 Sep;15(9):778–88. https://doi.org/10.1111/j.1442-2042.2008.02109.x PMID: 18643858
- Yokoyama O, Yotsuyanagi S, Akino H, Moriyama H, Matsuta Y, Namiki M. RNA synthesis in pons necessary for maintenance of bladder overactivity after cerebral infarction in rat. J Urol. 2003 May;169(5):1878–84. https://doi.org/10.1097/01.ju.0000052371.19582.5a PMID: 12686866
- Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Changes in dopaminergic and glutamatergic excitatory mechanisms of micturition reflex after middle cerebral artery occlusion in conscious rats. Exp Neurol. 2002 Jan;173(1):129–35. https://doi.org/10.1006/exnr.2001.7833 PMID: 11771945
- Ueki K. Disturbances of micturition observed in some patients with brain tumour. Neurol Med Chir (Tokyo). 1960;2(1-2):25–33. https://doi.org/10.2176/nmc.2.25
- Andrew J, Nathan PW. Lesions on the anterior frontal lobes and disturbances of micturition and defaecation. Brain. 1964 Jun;87(2):233–62. https://doi.org/10.1093/brain/87.2.233 PMID: 14188274
- Andrew J, Nathan PW. The cerebral control of micturition. Proc R Soc Med. 1965 Jul;58(7):553–5. https://doi.org/10.1177/003591576505800733 PMID: 19994438
- Sakakibara R, Hattori T, Yasuda K, Yamanishi T. Micturitional disturbance after acute hemispheric stroke: analysis of the lesion site by CT and MRI. J Neurol Sci. 1996 Apr;137(1):47–56. https://doi.org/10.1016/0022-510X(95)00322-S PMID: 9120487
- Tateno F, Sakakibara R, Yano M, Takahashi O, Sugiyama M, Kishi M, et al. A young man with herpes simplex encephalitis: andrew and Nathan type urodynamic abnormality. Bladder (San Franc). 2014;1(1):e4. https://doi.org/10.14440/bladder.2014.25.
- Sakakibara R, Hattori T, Yasuda K, Yamanishi T. Micturitional disturbance and the pontine tegmental lesion: urodynamic and MRI analyses of vascular cases. J Neurol Sci. 1996 Sep;141(1-2):105–10. https://doi.org/10.1016/0022-510X(96)00079-2 PMID: 8880701
- Kreydin EI, Gad P, Gao B, Liu CY, Ginsberg DA, Jann K. The effect of stroke on micturition associated brain activity: A pilot fMRI study. Neurourol Urodyn. 2020 Nov;39(8):2198–205. https://doi.org/10.1002/nau.24473 PMID: 32761953
- Sakakibara R, Tateno F, Aiba Y, Ogata T, Terada H, Inaoka T, et al. Prevalence of triple/dual disease (Alzheimer’s disease, Lewy body disease, and white matter disease). Neurol Clin Neurosci. 2020;8(4):171–6. https://doi.org/10.1111/ncn3.12377.
- Tateno F, Sakakibara R, Ogata T, Kishi M, Tsuyusaki Y, Takahashi O, et al. Lower urinary tract function in dementia with Lewy bodies (DLB). Mov Disord. 2015 Mar;30(3):411–5. https://doi.org/10.1002/mds.25985 PMID: 25356960
- Kono M, Nakamura Y, Ishiura Y, Komatsu K, Kontani H, Namiki M. Central muscarinic receptor subtypes regulating voiding in rats. J Urol. 2006 Jan;175(1):353–7. https://doi.org/10.1016/S0022-5347(05)00004-2 PMID: 16406941
- Sakakibara R, Ogata T, Aiba Y, Tateno F, Uchiyama T, Yamamoto T. Does depression contribute to the bladder and bowel complaint in Parkinson’s disease patients? Mov Disord Clin Pract (Hoboken). 2020 Dec;8(2):240–4. https://doi.org/10.1002/mdc3.13124 PMID: 33553494
- Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 1999 Nov;67(5):658–60. https://doi.org/10.1136/jnnp.67.5.658 PMID: 10519875
- Hanyu H, Shimuzu S, Tanaka Y, Takasaki M, Koizumi K, Abe K. Cerebral blood flow patterns in Binswanger’s disease: a SPECT study using three-dimensional stereotactic surface projections. J Neurol Sci. 2004 May;220(1-2):79–84. https://doi.org/10.1016/j.jns.2004.02.011 PMID: 15140610
- Richter N, Michel A, Onur OA, Kracht L, Dietlein M, Tittgemeyer M, et al. White matter lesions and the cholinergic deficit in aging and mild cognitive impairment. Neurobiol Aging. 2017 May;53:27–35. https://doi.org/10.1016/j.neurobiolaging.2017.01.012 PMID: 28208063
- Tadic SD, Griffiths D, Murrin A, Schaefer W, Aizenstein HJ, Resnick NM. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. Neuroimage. 2010 Jul;51(4):1294–302. https://doi.org/10.1016/j.neuroimage.2010.03.016 PMID: 20302947
- Kuchel GA, Moscufo N, Guttmann CR, Zeevi N, Wakefield D, Schmidt J, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009 Aug;64(8):902–9. https://doi.org/10.1093/gerona/glp037 PMID: 19386575
- Sakakibara R, Uchida Y, Ishii K, Kazui H, Hashimoto M, Ishikawa M, et al. the members of SINPHONI (Study of Idiopathic Normal Pressure Hydrocephalus On Neurological Improvement). Correlation of right frontal hypoperfusion and urinary dysfunction in iNPH: A SPECT study. Dement Geriatr Cogn Disord. 2011;32:1–10. https://doi.org/10.1159/000328972.
- Sakakibara R, Uchida Y, Ishii K, Hashimoto M, Ishikawa M, Kazui H, et al.; Members of SINPHONI (Study of Idiopathic Normal Pressure Hydrocephalus On Neurological Improvement). Bladder recovery relates with increased mid-cingulate perfusion after shunt surgery in idiopathic normal-pressure hydrocephalus: a single-photon emission tomography study. Int Urol Nephrol. 2016 Feb;48(2):169–74. https://doi.org/10.1007/s11255-015-1162-2 PMID: 26578001