Introduction
Imaging approaches to upper urinary tract (UUT) pathology, whether urolithiasis, tumors, malformations, iatrogenic injuries, or other disease, and follow-up on conservatively treated UUT tumors may involve both endoscopy and cross-sectional imaging (computed tomography, CT, or magnetic resonance imaging, MRI) to detect and characterize lesions and determine their extent. However, despite recent technical advancement in retrograde flexible ureteroscopy, such as increased flexibility [
1,
2], digitization [
1,
3], narrow-band imaging [
3], miniaturization of instruments, and modular concepts for ancillary equipment [
4], that now permit access to the entire renal pelvicocaliceal system [
1,
3], improve lesion detection [
3], decrease the risk of infection, and make maintenance both safer for the endoscope and less expensive [
4], endoscopy is still limited to surface views of the urothelium and its pathologies. Cross-sectional imaging on the other hand reliably provides information on tissue status around the UUT [
5,
6], but fails to distinguish between different histological wall layers. Thus, there remains a diagnostic gap between the surface view and the view beyond the urinary tract. However, since clinical decisions have to be made between endoscopic or open, conservative or radical surgical approaches to UUT lesions, particularly in malignant tumors [
1], pre-therapeutic information on different anatomical UUT wall layers around lesions may be critically important.
Optical coherence tomography (OCT) applies coherent near infra-red light (NIR, wavelength range, 800–1400 nm) to acquire cross-sectional images of biological tissues [
7-
15]. Depth of penetration is limited to about 2 mm in non-translucent biological tissues, such as human skin or vessel walls that strongly scatter NIR light [
11-
13]. Catheter-mounted OCT devices investigate hollow organs and vessels from inside their lumen in real time, with a spatial resolution of less than 20 µm [
7,
11-
14]. OCT applications in urology have concentrated on the urinary bladder. Normal urothelium demonstrates with several distinct layers, while pathologic conditions are recognized by a loss of distinct layering [
15-
17]. OCT may guide bladder biopsies [
18]. Extracorporal OCT demonstrates UUT tissue microstructure
post mortem [
10]. Intraluminal, cross-sectional OCT of porcine upper ureters
ex vivo, as performed with a miniaturized rotating catheter probe, reliably distinguishes between different anatomical layers, including the urothelium, lamina propria, and muscle layer [
7-
9].
We prospectively investigated the technical success of performing catheter-based OCT of the normal human UUT
in vivo through a rigid cystoscope and ureter catheter (UK), or through the working channel of a rigid ureteroscope. Distinction by OCT of different anatomical wall layers of the normal human UUT
in vivo was determined and compared with a previously established
ex-vivo porcine model [
7-
9], to estimate if a loss of OCT imaging information has to be expected in the clinical setting. The OCT imaging appearance of UUT lesions incidentally encountered in human UUTs
in vivo was described
.Patients, Material, and Methods
Ethical considerations
The study protocol was approved by the ethics committee at the faculty of medicine of the local university. Written informed consent for all urological procedures, including OCT, was obtained from each patient.
Optical coherence tomography (OCT)
Intraluminal, two-dimensional, cross-sectional OCT involved off-label use of a catheter-mounted OCT probe certified for
in-vivo examinations of human coronary arteries (“M2” OCT-system, LightLab Imaging, Inc., Westford, MA, USA), with spatial resolutions of approximately 10 × 15 µm (axial plane), and 25 µm (through-plane). The OCT system includes a coherent NIR light source (wavelength, 1300 ± 20 nm), and a beam splitter that directs half of the light into the measurement arm and half into the reference arm of a Michelson-type interferometer [
7,
10]. In the measurement arm, an electric drive rotates the 2.0-m-OCT-probe that includes a 400-µm optical fibre and a miniature mirror at the tip to emit and receive NIR light. Fibre integrity and mirror position are checked by a visible pilot light. Individual OCT images were obtained at different probe positions and displayed on the system monitor, either as grey-scale images or in shades of sepia, both during OCT measurements and after retrieval of data previously archived in compression-free .tiff format.
OCT in patients
A volunteer sample of 15 patients (5 female, 10 male, age+/-standard deviation, 52+/-17 years) underwent intraluminal OCT as part of an UUT investigation. Indications included suspicion of urothelial carcinoma (UC) in 4, urolithiasis in 10, and urinary obstruction in 2, the latter including one of the urolithiasis patients. Prior to OCT, retrograde uretero-pyelography (RUP) and/or uretero-renoscopy (URS) with biopsy were performed. In 7 patients, the sterile, single-use OCT probe was inserted into the UUT through a commercially available rigid cystoscope and ureteral catheter (UK, Selectip UK CH4 or CH5, Bard/Angiomed, Germany), and in the other 8, through a rigid CH10 ureteroscope with two working channels (No. 8703 523 or No. 8703 524, Richard Wolf, Knittlingen, Germany), alongside a wire guide. Only one UUT per patient was examined by OCT. Depending on time constraints in the operating room, 5 to 40 cross-sectional OCT images were acquired per patient. OCT added 5 to 10 minutes to urologic endoscopy, with the OCT scanner ready and the sterile OCT probes prepared to insert and plug into the OCT drive.
OCT of porcine upper ureter specimens
Eleven porcine kidneys with 7–10 cm of adherent upper ureter each were obtained fresh from the municipal slaughter house, immediately transported to the laboratory in air-tight containers at ambient pressure and temperature, and thoroughly rinsed, inside and out, with normal saline solution. Adherent tissues were removed to expose the renal hilus and upper ureter. Specimens were fixed to a Styrofoam pad placed in a shallow plastic tub, such that the respective long axis of the ureter and kidney were orthogonal to each other and there was easy horizontal access. The distal cut edge of the ureter was identified, and a 7F catheter sheath with a dilator and a side port for flush lines (Schleusen-Set 7F, Peter Pflugbeil GmbH Medizinische Instrumente, Zorneding, Germany) was inserted after flushing with normal saline solution. A size-1 (4 Ph. Eur.) surgical suture (Ethicon Ethibond Excel, Johnson & Johnson Intl.) was tied around the ureter, approximately 3 mm proximal to the cut edge, to secure the position of the catheter sheath. The dilator was removed, and a 10-ml syringe (Terumo Syringe, Terumo Europe N.V., Leuven, Belgium) with normal saline solution was connected to the side port of the catheter sheath with a three-way stop cock (Discofix-3, B. Braun Melsungen AG, Melsungen, Germany) whose third way remained open for draining. For OCT, the urinary collecting system was filled with normal saline solution, such that the cross-sectional diameter of the proximal porcine ureter was 3–4 mm, and the OCT catheter was inserted through the catheter sheath. Respective OCT probe positions were marked by the pilot light, which showed through the bare ureter wall.
Evaluation and statistics
Sample size calculation for dichotomized data in two-by-two tables [
19] assumed 89% delineation of wall layers in porcine upper ureter
ex vivo [
8], and 79% or less in human ureter
in vivo, 30% porcine and 70% human samples, since porcine upper ureters
ex vivo were considerably shorter than human ureters
in vivo, alpha-error 0.05 or less, and a power of 90% [
19], such that at least 204 porcine and 476 human samples had to be obtained to make a loss of OCT delineation of any UUT wall layers of at least 10%, from 89% to 79% or less, a statistically significant finding.
OCT images were evaluated in a consensus reading by two observers, including one attending urologist with longstanding experience in urologic endoscopy, and one attending radiologist with fellowship training in urogenital and abdominal imaging. To account for different distances of the OCT probe from the ureter wall that could potentially influence image quality, the analysis was based on the respective right and left upper and lower quadrants of each cross-sectional OCT image (statistical samples). For each quadrant, observers agreed if any wall layers, urothelium and lamina propria, lamina propria and muscle layer, different muscle layers, and different cell layers within the urothelium, respectively, did or did not delineate. Respective delineation in human UUT
in vivo and porcine UUT
ex vivo was compared applying a continuity-corrected chi-square-test [
20] at a significance level of
P < 0.05. UUTs with evidence of pathology at RUP or URS were excluded from the evaluation, but OCT findings were recorded in these cases.
Results
OCT access to the upper urinary tract
OCT was technically successful and acquired images in all human UUTs examined in vivo (Fig. 1). Respective OCT probe positions within the ureter immediately visualized through the rigid ureteroscope, such that ureteroscopy could guide OCT image sampling. However, neither the tip of the OCT probe nor the pilot light was visible to the endoscopist when applying the UK.
Figure 1. Axial, cross-sectional OCT image of human upper ureter in vivo demonstrates OCT probe (double-lined white arrow) in the center and wire guide of ureterorenoscope (triple-lined grey arrow) on the side of the ureter lumen.
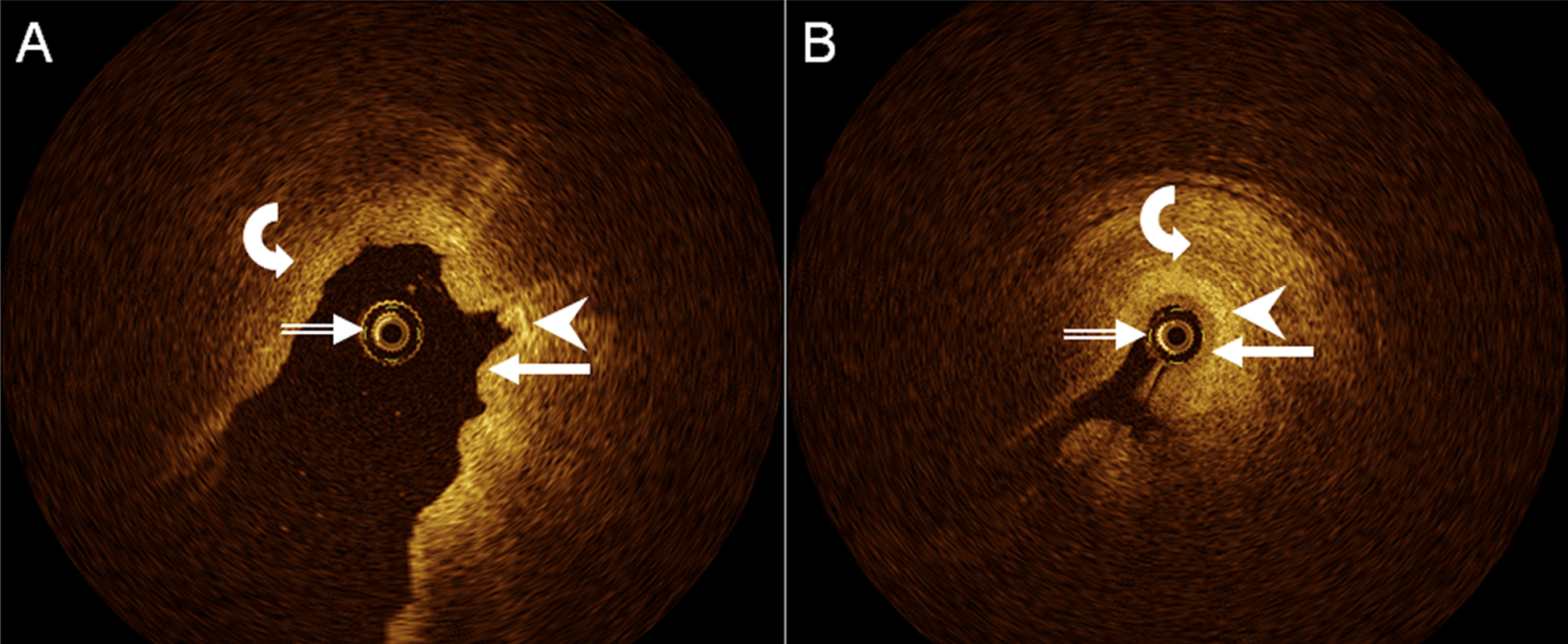
Figure 2. Axial, cross-sectional OCT images of human upper ureter in vivo, A, with fluid distending the ureter lumen, and B, with little fluid within the ureter lumen, demonstrate OCT probe (double-lined white arrows), urothelium (arrows), lamina propria (arrowheads), and muscle layer (curved arrows). Distinct layering of ureter wall appears to be better appreciated in (B).
OCT of the human upper urinary tract in vivo
In all, 532 OCT samples were obtained in 12 different human ureters in vivo, with a range of 20 to 160 samples per patient. In detail, any UUT wall layers delineated in 454 samples (85%; inter-patient range, 68–100%), urothelium and lamina propria, i.e., the first and second layer visualized from inside the ureter lumen, in 362 samples (68%; inter-patient range, 50–96%), and lamina propria and muscle layer i.e., the second and third layer, and moderate for urothelium and lamina propria, in 445 samples (84%; inter-patient range, 61–100%; Fig. 2). Delineation of different UUT muscle layers was modest (191 samples, 36%), with a very wide inter-patient range (5–89%). Delineation of different urothelial cell layers was poor (17 samples, 3%; inter-patient range, 0–14%).
However, there was a wide range between individual patients in the respective proportions of delineations, which did not appear to depend on patient age, gender, or mode of endoscopic access, but may have been related to individual filling state of the UUT lumen and respective distance of the OCT probe from the UUT wall, at least in some instances (Fig. 2).
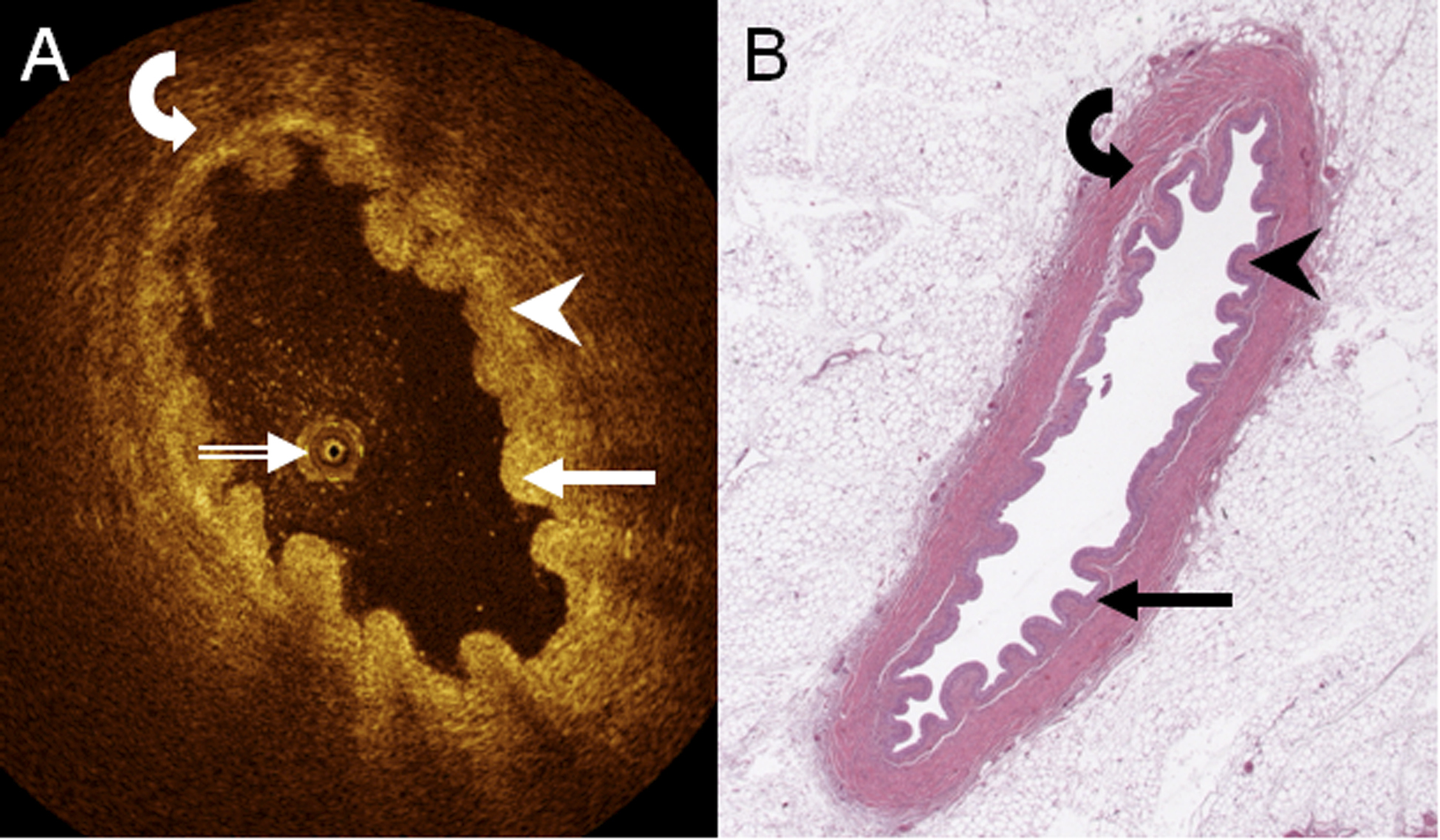
Figure 3. A. axial, cross-sectional OCT image of porcine upper ureter ex vivo, and B. corresponding digital light microscopy slide (H&E staining, low power, resolution appr. 4 μm) demonstrate urothelium (arrow), lamina propria (arrowhead), and muscle layer (curved arrow). The OCT probe shows in A (double-lined white arrow).
OCT - comparative delineation in the human and porcine upper urinary tract
When comparing the 532 (68.2% of all samples) normal in-vivo human samples (Fig. 1 and Fig. 2) with 248 (31.8%) ex-vivo porcine OCT samples obtained (Fig. 3), there were no statistically significant differences in the distinction of any wall layers (porcine, 223, 90%, chi-square, 2.898, P = 0.089) and the distinction of lamina propria and muscle layer (porcine, 200, 81%, chi-square, 0.865, P = 0.352). However, porcine OCT samples significantly more often delineated urothelium and lamina propria (193, 78%, chi-square, 7.409, P = 0.006), different muscle layers (207, 84%, chi-square, 151.25, P < 0.001), and different cell layers within the urothelium (172, 69%, chi-square, 399.67, P < 0.001).
OCT of the human upper urinary tract in vivo showing pathological findings
Three human ureters were excluded from the analysis of normal findings because RUP, URS, and biopsy detected stage Ta papillary-exophytic urothelial carcinoma. OCT showed stage Ta urothelial carcinoma as papillary extensions from the urothelium with intermediate brightness, with no apparent alteration of other layers of the UUT wall (Fig. 4). There was no case of invasive urothelial carcinoma.
Discussion
The most important findings are that intraluminal, cross-sectional OCT is technically feasible in the human UUT in vivo, may demonstrate urothelium, lamina propria, and muscle layer, and may recognize superficial urothelial carcinoma.
It has previously been shown for the urinary bladder that OCT distinguishes between different wall layers, both
ex vivo [
10] and
in vivo [
16,
17]. Suggested applications of OCT in the urinary bladder focus on urothelial carcinoma (UC) [
16,
17].
However, there is potential clinical interest to explore the capabilities of OCT in the UUT, too. Besides primary UC affecting the UUT, both recurrent and metachronous UC in the UUT have to be considered [
21]. Recently, the previously accepted standard of radical nephroureterectomy (RNU) for all stages of upper tract UC has been questioned. Nephron-sparing surgery (NSS) has been suggested as an alternative for patients with early stages of disease [
22,
23]. Reasons for NSS included marked differences in 5-year survival [
23] and cancer-related mortality [
24] between non-invasive and invasive UC of the UUT. Among 34 renal units managed endoscopically for low-grade UC of the UUT, cancer-specific and metastasis-free 5-year survival rates were 100% and 94%, respectively. However, 84% of patients had at least one ipsilateral recurrence [
25]. A comparative review of outcomes in 131 patients with UC of the UUT demonstrated a recurrence risk three times as high for NSS than for RNU. Authors suggested that extreme care should be taken to rule out occult invasive tumor prior to therapy and to follow up patients with NSS for upper tract UC closely [
23]. Also, in patients with UC affecting the bladder and at least one UUT, or in panurothelial disease, therapeutic concepts try to be as radical as necessary while preserving as much urinary tract function as possible [
26,
27]. In addition, extra-urinary tumors may compress or invade the UUT and become the subject of pre-therapeutic imaging [
28].
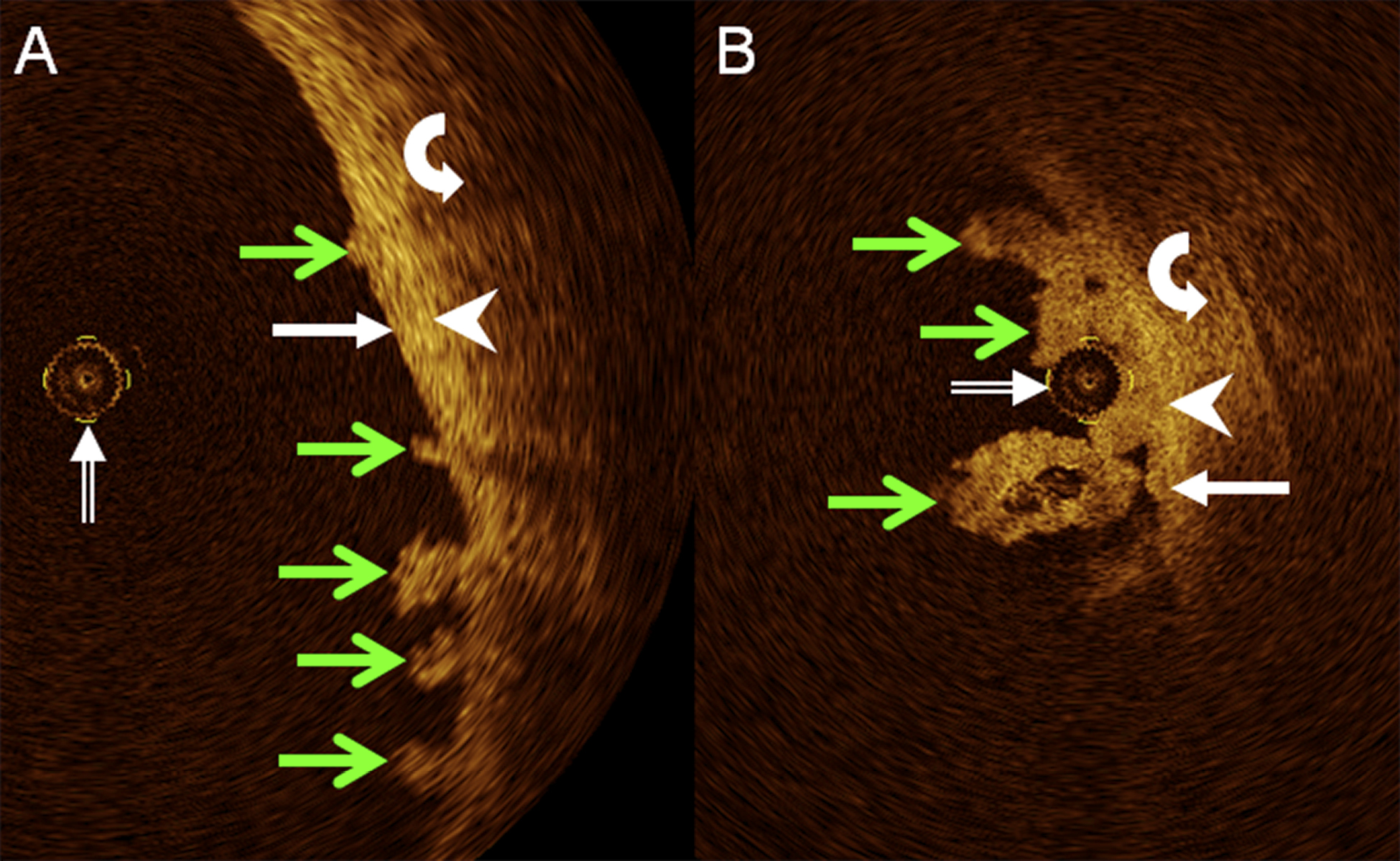
Figure 4. Details from axial, cross-sectional OCT images of human ureter in vivo in two different patients demonstrate superficial papillary-exophytic urothelial carcinoma (green arrows in A and B) remote from (A) or adjacent to (B) OCT probe (double-lined white arrow). Respective delineation of urothelium (white arrows), lamina propria (white arrowheads), and muscle layer (curved white arrows) remains undisturbed.
Among the 15 patients examined here, the catheter-borne OCT probe, which had originally been designed and certified by health authorities for intraluminal use in the human coronary arteries in vivo, was safely delivered to and guided within the UUT by means of both a rigid ureteroscope and a rigid cystoscope and UK. This may prove that intraluminal OCT is possible in the human UUT in vivo by established urological access routes, with OCT equipment already certified for clinical use and commercially available, if for different purposes and without optimization for specific urologic requirements. The OCT probe was more easily guided by ureteroscopy, because it immediately visualized respective OCT probe positions. For clinical practice, ureteroscopy may be the means to detect a lesion, or find the level of an extra-urinary lesion detected at CT or MRI, while OCT could be a means to explore the involvement of different UUT wall layers and thereby obtain prognostic information.
Distinction of three wall layers by means of OCT, as described previously [
7-
9,
29] and corroborated here for normal porcine UUT
ex vivo, was established, too, for normal human UUT
in vivo. When compared with normal porcine UUT, OCT of human UUT demonstrated both any wall layers and distinction between lamina propria and muscle layer equally well, at no apparent loss of image quality. However, distinction of urothelium and lamina propria, and of cell layers within the urothelium were significantly better in normal porcine UUT
ex vivo than in normal human UUT
in vivo. Although it has previously been shown by histological analysis that the normal porcine upper urinary tract closely resembles the normal human upper urinary tract in its morphological structure, including the presence of four to five layers of urothelium, one distinctive difference lies in the inclusion of mucinous cells within the urothelium in porcine, but not in human UUT [
30]. We cannot provide immediate evidence of an effect of the presence of mucinous cells on the ability of OCT to distinguish between the urothelium and lamina propria or between different urothelial cell layers. However, indirect evidence may derive from the fact that the lamina propria and muscle layer delineated equally well in the porcine model and in human UUT, such that anatomical differences in urothelial structure appear to be a more likely reason than technical differences between porcine and human OCT studies. It may be hypothesized that one factor contributing to the superior delineation by OCT of different UUT muscle layers in the porcine model was its
ex-vivo state, without ureteral peristalsis, when compared to human UUT
in vivo.
There was considerable variation between individual patients in the distinction of urothelium and lamina propria, but also of lamina propria and muscle layer. While differences in peristaltic activity of the ureter may be one explanation, potential influence of previous intraluminal implants or urologic devices on urothelial microstructure [
30] may be another. However, in this study, no data were collected to support or rule out these hypotheses. Still other reasons may have been different filling states of the UUT at the respective levels of OCT imaging, whether due to peristalsis or due to the endoscopic procedure, with resulting differences in the distance between the tip of the OCT probe and the UUT wall.
Incidental OCT findings in three patients with superficial, papillary-exophytic UC of the UUT proven by RUP, URS, and biopsy showed lesions clearly confined to the innermost wall layer visible, with the other wall layers apparently unchanged. While findings emphasize the essential capability of intraluminal OCT to recognize superficial UC in the UUT as being non-invasive, the number of cases is currently too small to infer to the clinical staging capacities of OCT in the UUT. In particular, if lesions were to involve oedema, or inflammatory or tumorous expansion of one or more wall layers of the ureter, the respective deeper wall layers could be further separated from the NIR light source of the OCT system, possibly beyond the transmission limit for OCT in non-transparent biological tissue [
10]. Our results match recent experience in seven of eight patients with UC of the UUT whose OCT findings of depth of tumour invasion were in accordance with histopathology, while tumour thickness exceeded the transmission limit for OCT in the eighth patient [
31].
Conclusions
Intraluminal OCT appears to be technically feasible in the human upper urinary tract in vivo, particularly when guided by ureteroscopy, and demonstrates three distinct anatomical wall layers, deemed to represent the urothelium, lamina propria, and muscle layer. Although delineation of lamina propria and muscle layer was similarly good in porcine ureter ex vivo and human ureter in vivo, delineation of urothelium and lamina propria differed, possibly due to anatomical differences in urothelial structure and to ureteral peristalsis in the latter. Although superficial urothelial carcinoma was recognized as being non-invasive by means of OCT in three patients, further studies in more patients have to confirm this observation.





