Introduction
Therapeutic options for patients suffering from the overactive bladder syndrome (OAB) are limited and often unsatisfactory. Therefore, new targets have to be identified, which might offer new therapeutic modalities. One of these potential targets could be the prostanoid system.
Urine levels of Prostaglandin E2 (PGE
2) are increased in patients suffering from the overactive bladder syndrome [
1,
2]. PGE
2 exerts its effect by binding one of four E prostanoid (EP) receptors, EP1–4. Morphological studies have shown that the EP1 and EP2 receptors are expressed in the urinary bladder throughout different tissue layers [
3,
4]. Administration of PGE
2 directly into the human urinary bladder causes strong sensations of urgency [
5]. In animals, intravesical administration of PGE
2 has been shown to provoke detrusor overactivity. It has been hypothesized that this is caused by activation of sensory bladder afferent neurons [
6-
9]. In addition, inhibition of EP1 and EP2 reduced the effect of PGE
2 dose-dependently in isolated guinea pig bladders [
10]. In vivo studies in mice have shown that intravesical administration of PGE
2 itself or an EP1 agonist reduced significantly the inter-micturition interval [
11]. Inhibition of the PGE
2 producing cyclooxygenase (COX) family inhibited the micturition reflex in rats with bladder overactivity which was less distinctive in control rats [
12].
At a molecular level, PGE
2 has been shown to induce the release of acetylcholine (ACh) in tissue preparations containing urothelium and lamina propria [
13]. On the other hand, stimulation with the muscarinic agonist arecaidine evoked PGE
2 release [
13]. Another study showed that administration of the non-selective COX inhibitor indomethacin to a whole bladder preparation reduced contractions evoked by the muscarinic agonist arecaidine
ex vivo [
14]. Hence, it can be hypothesized that PGE
2 exerts its effect on bladder activity by modulation of muscarinically induced contractions.
Acetylcholine is the primary neurotransmitter in the excitation-contraction coupling of the human detrusor muscle. Antimuscarinic drugs used in OAB treatment, inhibit the ACh binding on muscarinic receptors within the urinary bladder. The receptor subtypes M2 and M3 are expressed on detrusor smooth muscle cells, basal urothelial cells and suburothelial interstitial cells [
15-
17]. The high prevalence of side effects of antimuscarinics might be circumvented, if drugs are selected to not target the muscarinic system itself, but systems which modulate muscarinic induced contractions, such as the progstaglandin system. We hypothesize, that the prostaglandin system modulates muscarinic induced contractions
ex vivo, and that the EP1 receptor plays an important role herein. To investigate this, the effect of manipulations in the prostaglandin system on muscarinic induced contractions has been explored in this study.
Methods
Animals
A total number of 24 male guinea pigs were sacrificed by a percussive blow to the head, followed by exsanguination. All procedures were carried out with the approval of guidelines of the animal ethics committee of Maastricht University and were in line with European Community guidelines.
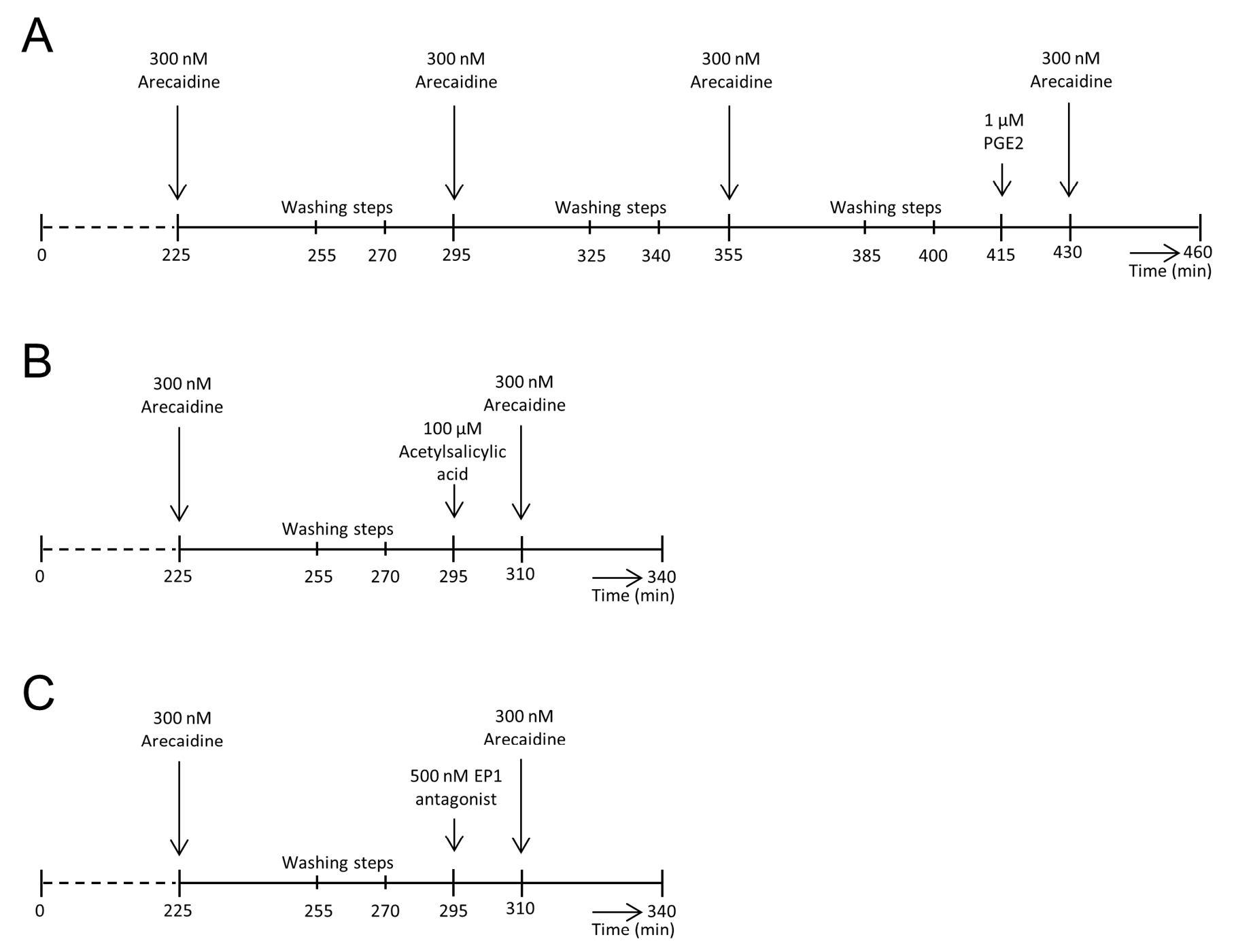
Figure 1. Overview of experimental groups and their protocols. A. Protocol used for bladders of the control group. After 3 stable and comparable arecaidine stimulations, each separated by two washing steps, PGE2 was added prior to a last arecaidine stimulation. B. Protocol for the COX-inhibition group. After a stable arecaidine response, two washing steps were performed. Subsequently, PGE2 production was inhibited by acetylsalicylic acid, followed by another arecaidine stimulation. C. Protocol for EP1 inhibition. Again, a standardized, comparable arecaidine response was generated. After two washing steps, bladders were incubated with an EP1 antagonist prior to a last arecaidine response. T = 0 on the time axis refers to the start of recording (approximately 30 min after sacrifice of the guinea pig).
Pressure recordings
The urinary bladder and proximal urethra were excised immediately after sacrificing the animal and placed in Krebs solution (mM: NaCl 121.1; KCl 1.87; CaCl2 1.2; MgSO4 1.15; NaHCO3 25; KH2PO4 1.17; glucose 11.0), aerated with 5% CO2 and 95% O2 (pH 7.4, 37°C). The bladder was emptied manually and the urethra was catheterized with a catheter made from PE-50 tubing with a small cuff at the end and fixed with a fine ligature. The bladder was then transferred to a heated organ bath (40 ml, 33–36°C) containing constantly aerated Krebs solution, and the catheter was connected through a fluid filled tube containing a three-way connector to a IBP pressure transducer with a pvb connector (Codan, Germany). The transducer output was amplified, digitized at 1000 Hz and recorded using a data capture system (MP150 with AcqKnowledge 4.2 software, BIOPAC systems Inc, California). The transducer was calibrated before each experiment. Recordings started 30 min after the animal was sacrificed. The pressure recordings started with an incubation period of 30 min. Subsequently, the bladder was filled to 1.5 ml in 1 hour and allowed to rest for another 60 min, followed by two washings steps, each lasting 15 min. During the washing steps, the Krebs solution in the organ bath was completely renewed by complete draining and refreshing. The bladder was stimulated for 30 min with the muscarinic agonist arecaidine but-2-ynyl ester tosylate (300 nM, Tocris, Avonmouth, UK). After two more washing steps, each lasting 15 min, this stimulation type was repeated in order to create a stable muscarinic response. From here on, protocols differed dependent on the experimental group. An overview of all protocols starting at the second arecaidine stimulation is given in Figure 1.
Experimental groups
In this study, three experimental groups were designed. To verify that subsequent arecaidine stimulations were comparable to each other, a control group (n = 8) was introduced. Bladders of the control group underwent, after the initial arecaidine response mentioned above, three consecutive stimulations with 300 nM, each followed by two washing steps. After three comparable, consecutive arecaidine stimulations, bladders were washed again by two washing steps and incubated for 15 min with 1 µM PGE2 (Sigma Aldrich, Missouri, USA), followed by a last stimulation with 300 nM arecaidine.
In a second experimental group (n = 8), the effect of inhibition of PGE2 production by the COX-inhibitor acetylsalicylic acid was investigated. After a first comparable arecaidine response, bladders were washed during two washing steps and incubated for 15 min with 100 µM acetylsalicylic acid. To investigate if PGE2 exerts its effect on muscarinic induced contractions by the EP1 receptor, a third experimental group was designed (n = 8). In this group, bladders were incubated with 500 nM of the EP1 antagonist after the first comparable arecaidine stimulation and subsequent washing steps. After 15 min of incubation, bladders were stimulated again with the muscarinic agonist arecaidine.
Drugs
Concentrated drug solutions were added directly to the organ bath to achieve the required final dilution. All drugs were added to the solution bathing the serosal surface. PGE2 (1 µM, Sigma Aldrich, Missouri, USA) was used to stimulate the bladders with exogenous PGE2. The PGE2 producing enzymes were inhibited by the general COX-inhibitor acetylsalicylic acid, used in a concentration of 100 µM (Sigma Aldrich, Missouri, USA). Acetylsalicylic acid was used as it blocks both PGE2 producing enzymes, COX-1 and COX-2. Furthermore, effects on other regulators are not known. The EP1 antagonist ONO-8713 (ONO pharmaceutical, Osaka, Japan) was used in a concentration of 500 nM. As muscarinic agonist, 300 nM of arecaidine but-2-ynyl ester tosylate (Tocris, Avonmouth, UK) was used. For all drugs, optimal concentrations were determined by dose-response curves.
Data processing and statistical analysis
All data were processed in a maximized objective way, using MATLAB scripts to detect peaks and corresponding baseline values. For peak selection a threshold of 5 cm H2O was used. To determine the moment of transition between the two phases, a strong low pass filter was deployed. This resulted in a simplified curve, starting with an ascending slope, which continues into a descending slope, shown in Figure 2. This part reflects the irregular rise in pressure during the first phase of an arecaidine response and is followed by an irregular sinus curve, reflecting the second phase. The first minimum of this curve was determined as the moment of transition towards the second phase of an arecaidine induced response (Fig. 2).
To analyze the effect of the drugs used to modify muscarinic induced contractions, the first comparable muscarinic response was used as reference and compared with subsequent responses after drug incubation. The effect was analyzed using the Kruskal-Wallis test and Wilcoxon matched pairs test.
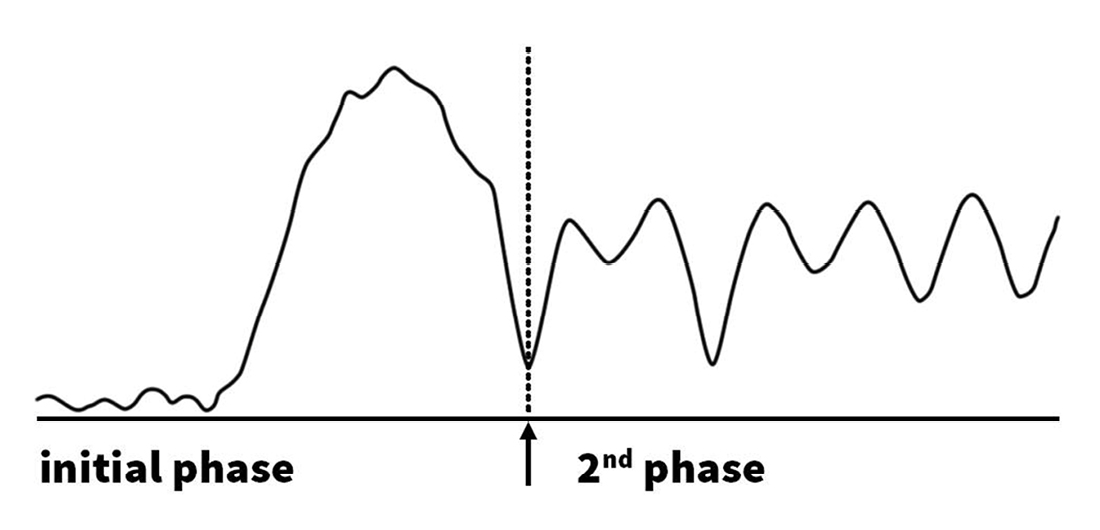
Figure 2. Separation of initial and second phases of arecaidine induced responses. The point of transition between the two phases was determined by deployment of a strong low pass filter. The simplified curve, resulting from the filtering process is shown in this figure. Afterwards, the first minimum after the strong rise was chosen as the point of transition from the initial phase towards the second phase.
Results
To ensure that consecutive arecaidine stimulations were comparable to each other, a control group was included. In this control group, bladders were subjected to four consecutive arecaidine stimulations. The first stimulation was used to standardize all bladders and therefore not taken into consideration during the analysis.
In general, the arecaidine response was divided into two phases. The initial phase was characterized by an irregular rise in pressure, low/mediate amplitude and high frequency contractions, and lasted for approximately 2-5 min. Afterwards, contraction trains with regular intervals developed. These phasic contractions were characterized by a higher amplitude and a lower frequency compared to the contractions of the initial phase (Fig. 3A). Due to distinct characteristic differences between these two phases, all responses were analyzed for frequency and amplitude of both the first and the second phases. In addition, the length of the initial phase has been registered. In the control group, no significant differences were measured in between the consecutive arecaidine stimulations (2nd, 3rd and 4th stimulations) for all parameters mentioned above (Fig. 3B-3F).
Subjectively, two different patterns in the arecaidine response were recognized after PGE2 stimulation. Some bladders showed an initial tonic increase in pressure followed by the low frequency phasic contractions, whereas others started with high frequency phasic contractions followed by similar low frequency contractions. Figures 4B-4E show typical examples of these two patterns. The two patterns were distributed more or less equally among the tested bladders. However, these differences in muscarinic responses were not detectable by the automated analysis. None of the analyzed parameters showed significant changes. A general overview of a tracing with both arecaidine responses (before and after PGE2 administration) is presented in Figure 4A.
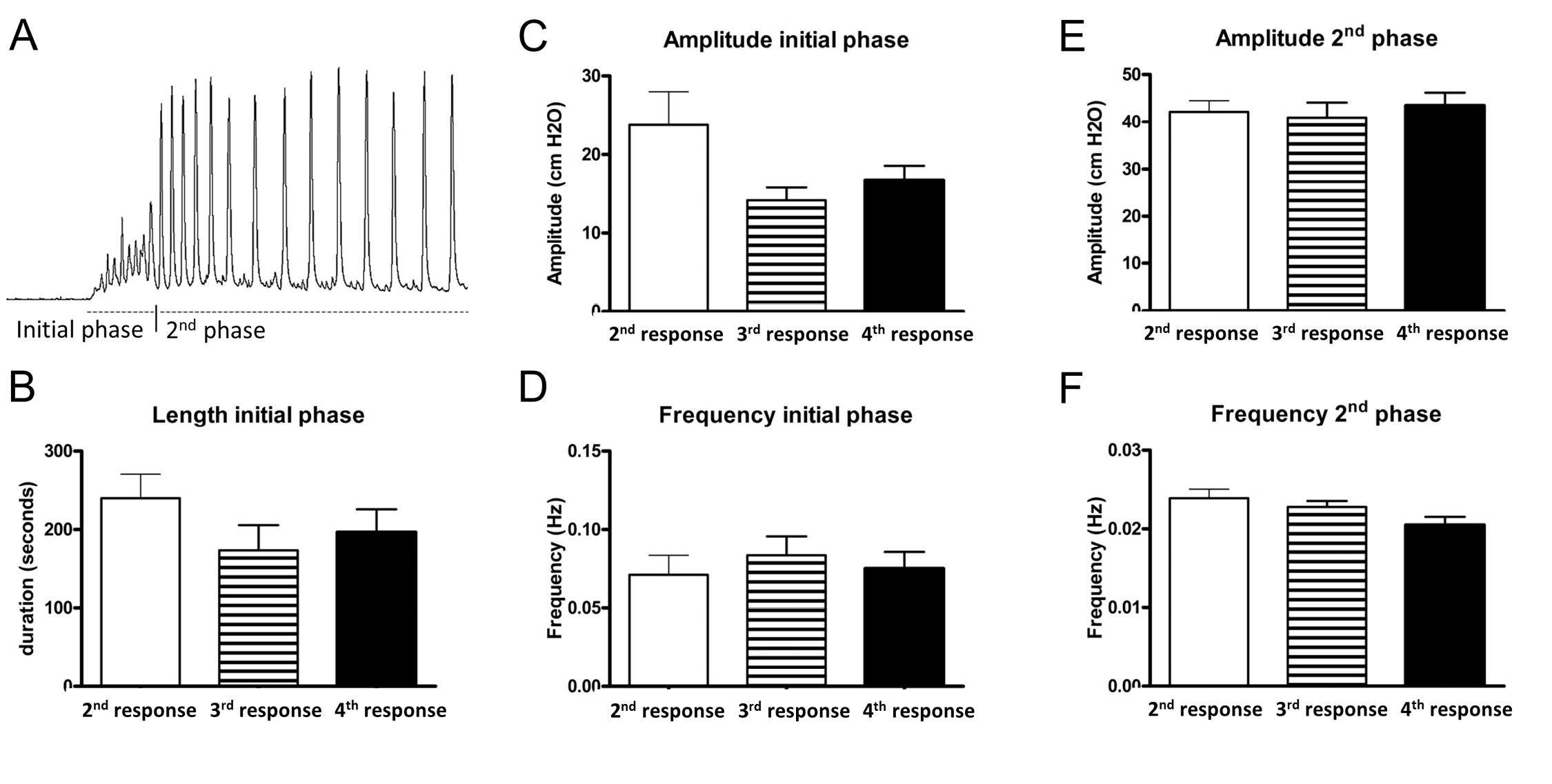
Figure 3. Characteristics and verification of muscarinic responses. A. Typical example of an arecaidine response. It could be divided into two phases. The initial phase was characterized by an irregular rise in pressure, low/mediate amplitude and high frequency contractions, and lasted for approximately 2–5 min. Afterwards, regular contractions developed. These phasic contractions were characterized by a higher amplitude and lower frequency compared to the contractions of the initial phase. B-F. Three consecutive arecaidine stimulations (2nd-4th stimulation). All stimulations were separated from each other by two washing steps. In none of the analyzed parameters significant changes could be detected between the consecutive stimulations. For each analyzed parameter, a different graph is shown.
Adding acetylsalicylic acid before stimulation with arecaidine resulted in a diminished rise in tonic pressure during the initial phase of the arecaidine response. In addition, the amplitude of the first phase of the muscarinic response decreased significantly after COX-inhibition. Figure 4F shows a typical example of how the rise in pressure and amplitude during the initial phase of the arecaidine response changes due to the inhibition of COX-1 and COX-2 enzymes. The amplitude during the initial phase of an arecaidine induced response decreased significantly after inhibition of the COX enzymes (P < 0.01, Fig. 4G)
In order to investigate whether the effect of PGE2 is exerted by binding to the EP1 receptor, bladders were incubated with an EP1 antagonist prior to the second comparable arecaidine stimulation. No significant changes were observed during the initial phase of the arecaidine induced response. However, during the second phase, the frequency of contractions decreased significantly (P < 0.01). No changes were detected regarding the amplitude during the second phase (Fig. 5).
Discussion
The aim of this study was to investigate whether muscarinically induced contractions can be modified by influencing the prostaglandin system. Adding PGE
2 before muscarinic stimulations induced an amplifying effect on muscarinically induced contractions. During these amplified contractions two different response patterns were seen. Due to that, the bladders could be divided into two groups. Remarkably, most of these bladders still had increased baseline activity after stimulation with PGE
2, comparable to those Rahnama’i
et al described in their study [
14]. Thus, PGE
2 still has an effect on baseline spontaneous activity of those bladders, even if it is not clearly visible in muscarinic evoked responses. In 2012, Nile
et al. already reported that PGE
2 and ACh act in a positive feedback manner on a molecular level. This was shown in preparations of bladder strips containing urothelium and lamina propria [
13]. Assuming that this positive feedback mechanism is also active in whole bladder preparations,
e.g. in the present study, it can be hypothesized that endogenous PGE
2, produced through this positive feedback has the same effect as exogenously administered PGE
2. Thus, PGE
2 seems to amplify muscarinically induced contractions. Responses could be divided into two distinct patterns of response after the amplification.
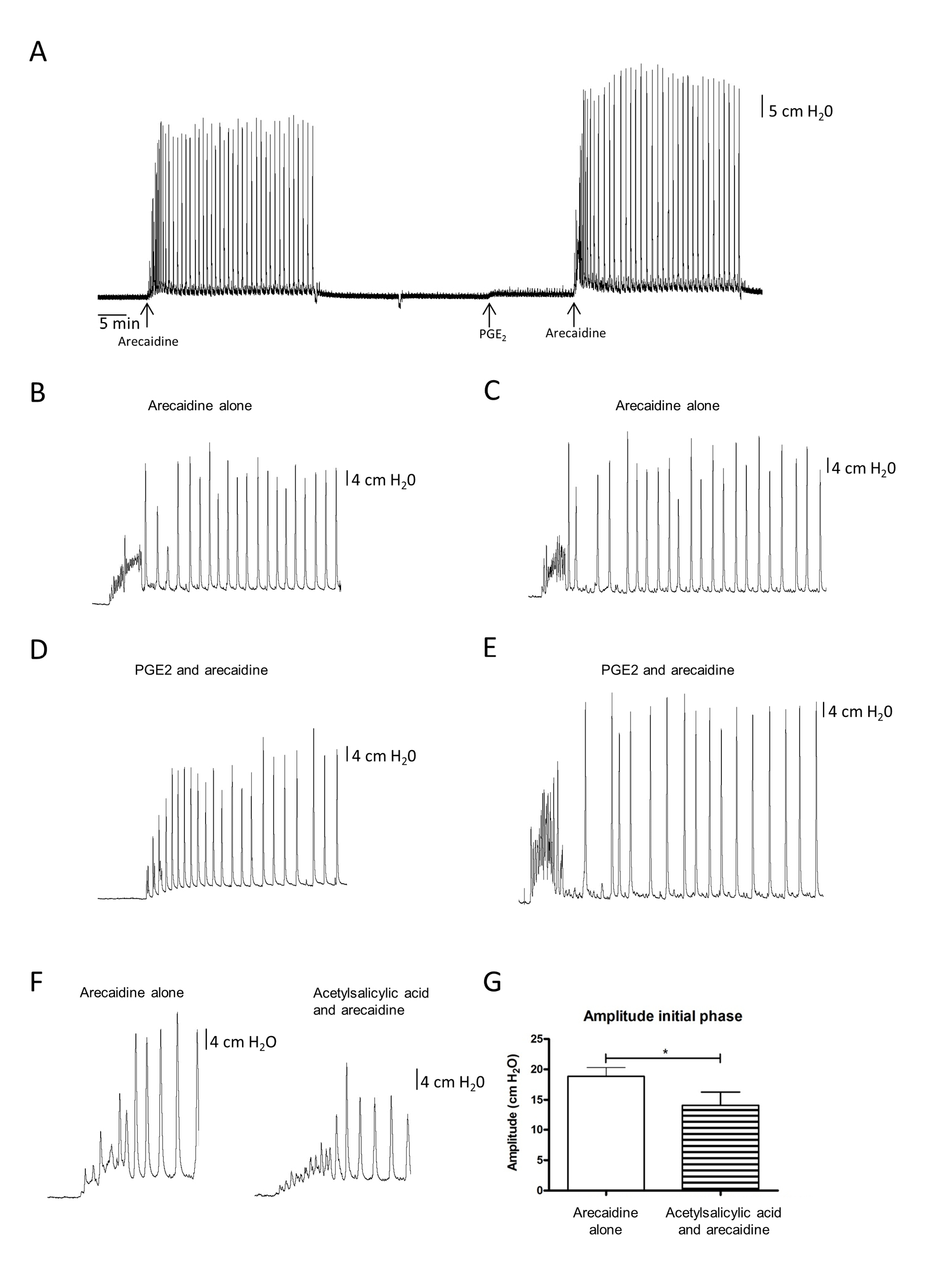
Figure 4. Effect of PGE2 and acetylsalicylic acid. B–E. Two different patterns observed during arecaidine stimulations after PGE2 stimulation (n = 8). Panel B and C show arecaidine induced responses before PGE2 stimulation. Arecaidine induced responses of the same bladders after PGE2 stimulation are shown in panel D and E, respectively. However, no significant differences could be detected using the automated analysis. In panel D, it seems that the bladder evolves faster into the second phase of the response, whereas in panel E, it seems that the initial phase of the response is reinforced. F and G. Effect of acetylsalicylic acid on the arecaidine induced response is shown (n = 8). Clearly the amplitude of the initial phase has been decreased. This effect is statistically significant (P < 0.01).
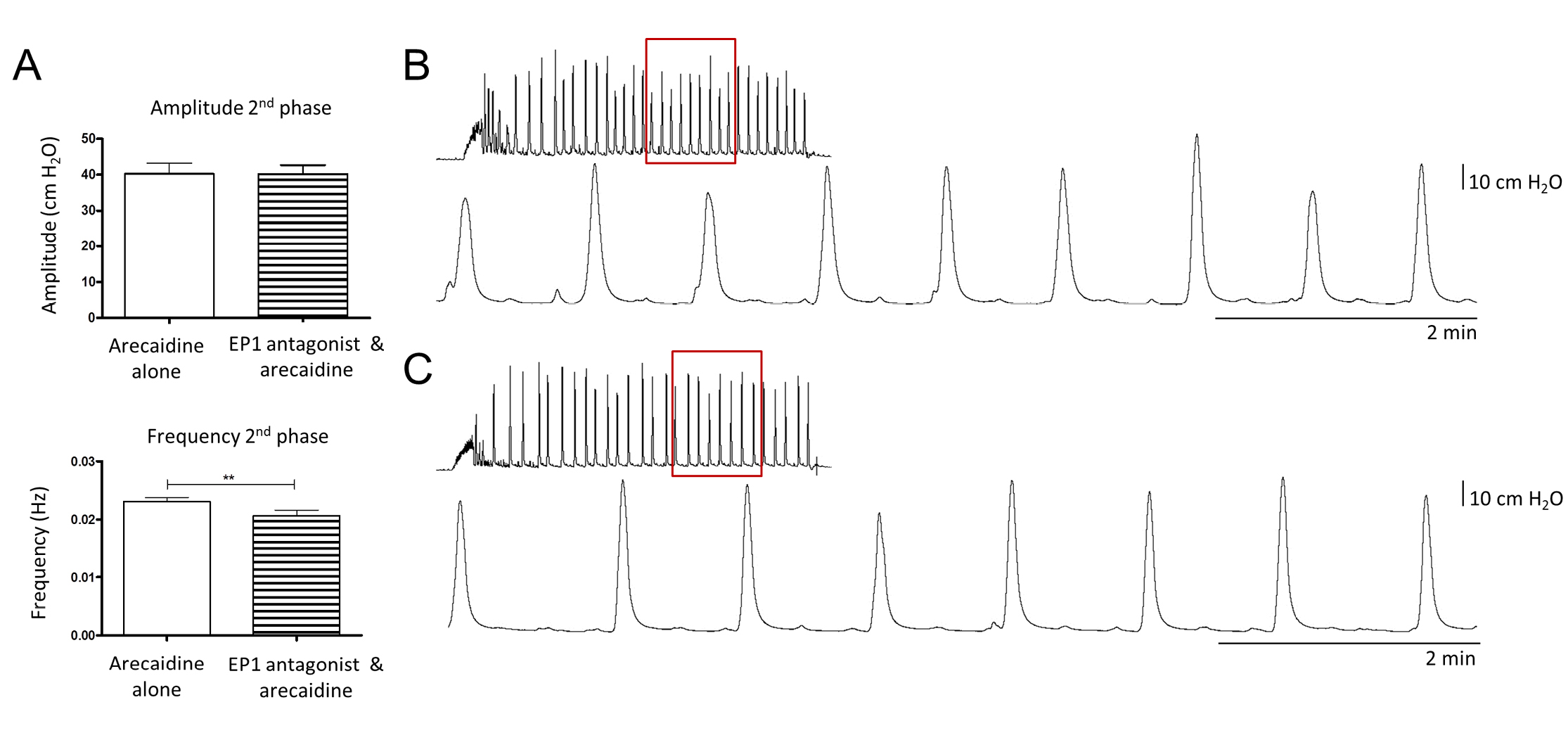
Figure 5. Effect of the EP1 antagonist ONO-8713 on muscarinic induced contractions. A. Quantitative data of the second phase of arecaidine responses after EP1 inhibition (n = 8). During this phase, frequency of contractions was reduced significantly (P < 0.01). No changes were detected in the amplitude of contractions during the second phase. B and C. Typical responses to arecaidine before (B) and after (C) EP1 inhibition.
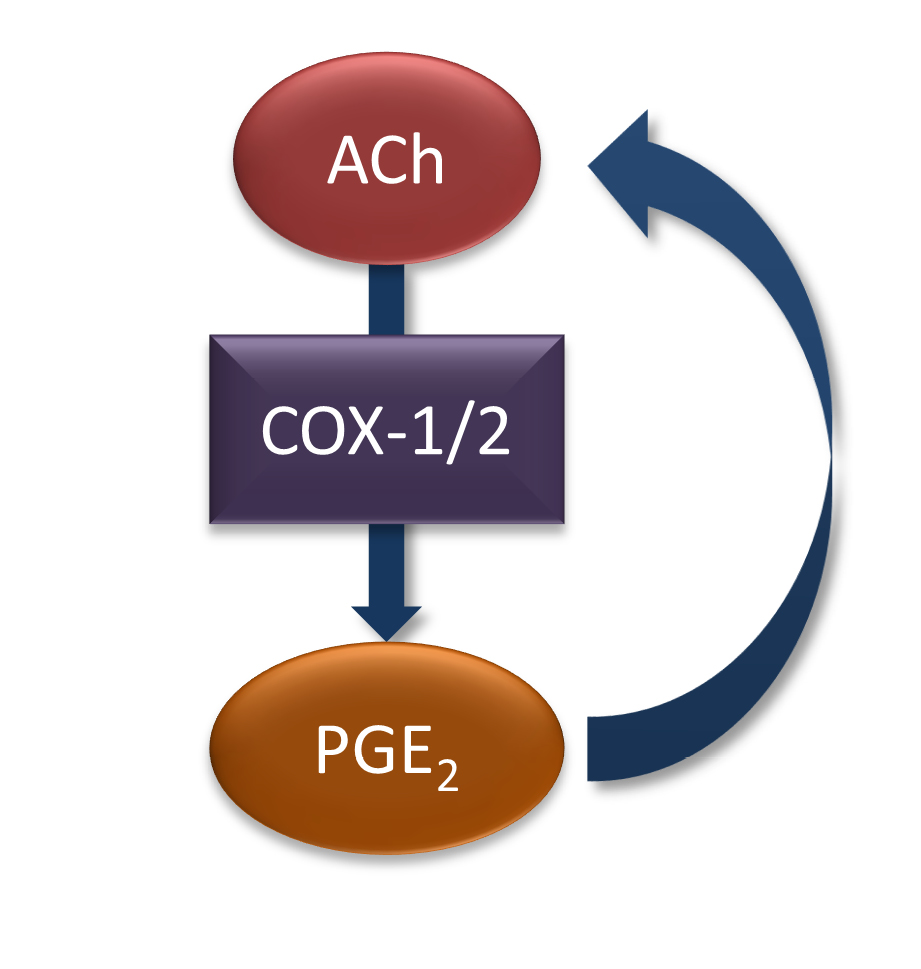
Figure 6. Positive feedback of ACh and PGE2 in the guinea pig urinary bladder. ACh activates the PGE2 producing enzymes COX-1 and/or COX-2. This results in an increase of PGE2 which in turn induces the secretion or release of more ACh. Via this pathway, a positive feedback takes place between the two signaling systems.
In addition, we showed that inhibition of the PGE
2 producing enzymes prior to an arecaidine stimulation decreased the amplitude during the initial phase of the response. Again, this supports the theory of a positive feedback mechanism between PGE
2 and ACh on a whole bladder/functional level. In a normal situation, muscarinic stimulation seems to activate COX enzymes, which will lead to the production of more PGE
2. PGE
2 in turn will promote the release of more ACh, that results in an amplified contraction (
Fig. 6). Because COX enzymes were inhibited during this part of the study, this feedback loop was interrupted, resulting in a diminished response. The feedback loop between the cholinergic and prostanoid system could be located within the urothelium and lamina propria, as it has been described in the study of Nile
et al. [
13]. Within these layers, both, ACh and PGE
2 are produced and the appropriate receptors are expressed [
3,
16,
18]. However, in our study, drugs were applied directly into the organ bath, stimulating the serosal side of the bladder. The PGE
2 specific receptor EP2 has been shown to be expressed in the muscle layer [
19]. As ACh is secreted by motor neurons, the cholinergic system is active within the muscle layer as well. Hence, it is likely, that the feedback loop, which was identified in our study, involves all different layers of the bladder wall.
Schroder
et al. showed that the EP1 receptor plays a role when PGE
2 exerts its effects on bladder activity [
8]. Therefore, an EP1 antagonist was administered to isolated bladders and its effect on arecaidine induced responses was tested. Inhibition of EP1 resulted in a decreased frequency during the second phase of the arecaidine response but the amplitude did not change. However, this effect was not comparable to the effect which was seen after inhibition of the PGE
2 producing COX enzymes by acetylsalicylic acid. This may reflect that other subtypes of PGE
2 specific receptors (EP2
-4) are involved in the signal transduction between PGE
2 and ACh. The exact mechanism has to be explored in future research in order to find a specific target which can be used to modulate muscarinic induced contractions and thereby avoid the high prevalence of side effects seen during current first line treatment regimens.
Conclusions
In summary, this study provides evidence that the cholinergic and prostanoid systems in the urinary bladder act in a positive feedback loop and that the EP1 receptor plays a subtle role in this process. In order to identify new treatment targets, the location of the different steps in the cascade and the targets for modulation of this process need to be determined in the near future.