Staphylococcus aureus urinary tract bacteriuria: single-institutional antibiotic susceptibility trends over a decade

Objectives: Methicillin resistant Staphylococcus aureus (MRSA) is a troublesome pathogen which is difficult for clinicians to treat. The purpose of this surveillance program is to assess the prevalence of MRSA urinary tract infections and determine risk factors for methicillin resistance in adults amongst urinary isolates of SA and to describe the antibiotic susceptibilities to guide empirical therapy.
Methods: From 2005 through to 2014, we retrospectively reviewed urine cultures recorded in a laboratory database at a university hospital in Cambridge, UK. Susceptibility testing was performed by BSAC (British Society of Antimicrobial Chemotherapy) disc diffusion testing and reported for fluoroquinolones, gentamicin, nitrofurantoin, linezolid, trimethoprim and vancomycin. Samples were denoted “MRSA” if they were resistant to oxacillin or cefoxitin.
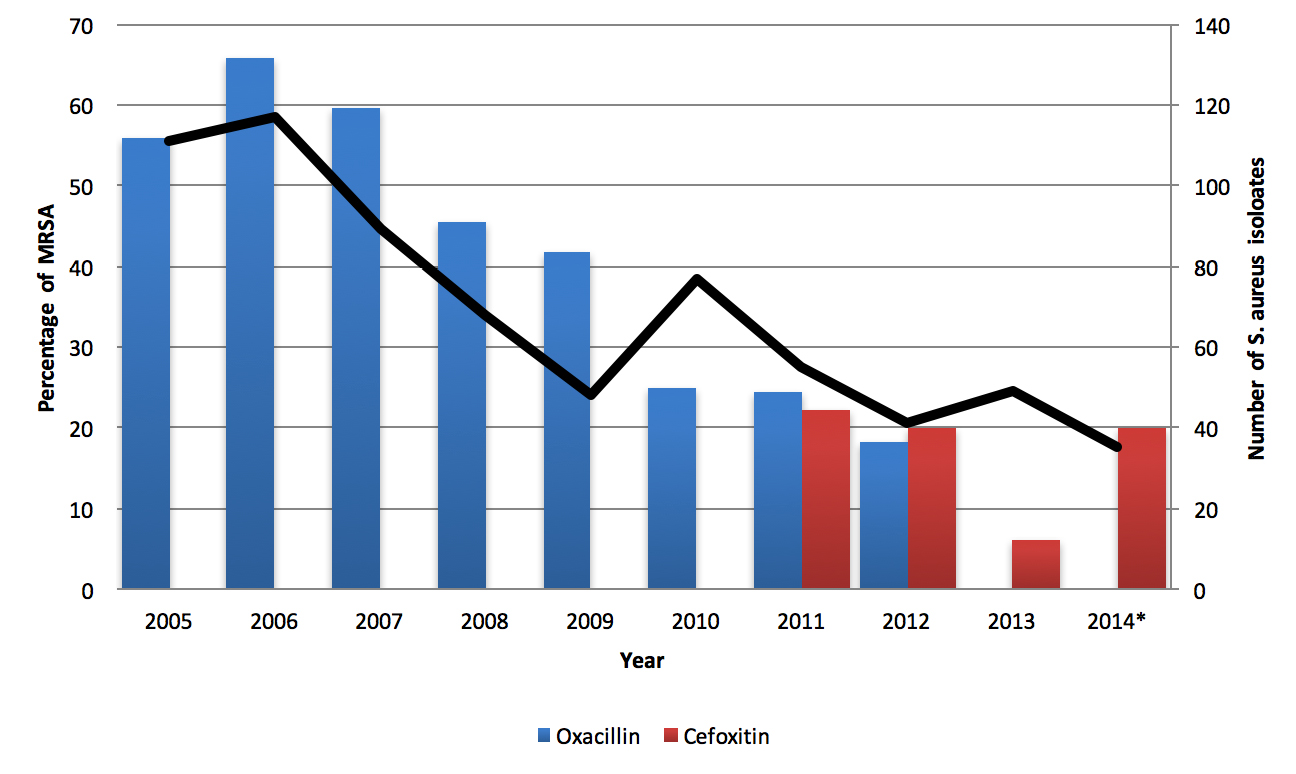
Results: In total, 690 cultures were positive for SA, of which 293 (42.5%) were methicillin resistant. The number of SA bacteriuria decreased from around 100 per year to 40 per year. The proportion demonstrating methicillin resistance decreased from around 60% to around 20%. Both methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA isolates were susceptible to vancomycin and nitrofurantoin. MRSA isolates demonstrated some increased resistance to trimethoprim and gentamicin and greatly increased resistance to fluoroquinolones. Urinary catheterization and increasing age were risk factors for methicillin resistance.
Conclusion: The incidence of SA and MRSA bacteriuria decreased during the study period. A high degree of resistance to fluoroquinolones was observed in MRSA compared to MSSA. Analysis of antibiotic susceptibility profiles suggests nitrofurantoin and trimethoprim may be useful in treating uncomplicated MSSA and MRSA urinary tract infections without concurrent bacteremia.
Introduction
Patients and Methods
Patient population
Culture analysis
Statistical analysis
Results
| 49864 isolates recorded in electronic database |
|---|
| Exclusion criteria applied |
| 37538 isolates remaining |
| 873 heavy mixed growth |
| 2197 missing age, gender or antibiotic susceptibility |
| 3094 aged < 16 years |
| 693 unusual specimen type |
| 5469 samples repeated within 30 d |
| 690 Staphylococcus aureus |
| 397 MSSA |
| 293 MRSA |
Discussion
| MSSA (%) | MRSA defined by oxacillin (%) | MRSA defined by cefoxitin (%) | P value for difference MSSA vs. all MRSA | P value for difference MRSA by oxacillin vs. cefoxitin | |
|---|---|---|---|---|---|
| Vancomycin | 277/277 (100) | 271/271 (100) | 2/2 (100) | ||
| Gentamicin | 392/397 (98.7) | 263/273 (96.3) | 17/20 (85.0) | 0.014 | 0.050 |
| Nitrofurantoin | 393/395 (99.5) | 271/273 (99.3) | 20/20 (100) | > 0.9 | > 0.9 |
| Trimethoprim | 375/395 (94.9) | 251/273 (91.9) | 16/20 (80.0) | 0.063 | 0.088 |
| Linezolid | 2/2 (100) | 1/1 (100) | 9/9 (100) | ||
| Nofloxacin/ciprofloxacin | 266/370 (71.9) | 10/266 (3.76) | 1/29 (3.45) | < 0.001 | > 0.9 |
| Gender n (%) | Catheter n (%) | Age n (%) | Total | ||||
|---|---|---|---|---|---|---|---|
| Female | Male | No | Yes | < 65 years | > 65 years | ||
| S. aureus | 232 (34) | 458 (66) | 441 (64) | 249 (36) | 218 (32) | 472 (68) | 690 |
| MSSA | 137 (35) | 260 (65) | 287 (72) | 110 (28) | 168 (42) | 229 (58) | 397 |
| MRSA | 95 (32) | 198 (68) | 154 (53) | 139 (47) | 50 (17) | 243 (83) | 293 |
| E. coli | 15630 (79) | 4057 (21) | 16580 (84) | 3107 (16) | 8548 (43) | 11139 (57) | 19687 |
| All pathogens | 25945 (69) | 11593 (31) | 29533 (79) | 8005 (21) | 15916 (42) | 21622 (58) | 37538 |
| Study (Year) | Isolate | Clinical setting | Staph. aureus (n) | MRSA (n) | MRSA (%) | Susceptible vancomycin (%) | Susceptible entamicin (%) | Susceptible fsluoroquinolones (%) |
|---|---|---|---|---|---|---|---|---|
| The present study | Urine | Hospital | 690 | 293 | 42 | 100 (MRSA) 100 (MSSA) | 96 (MRSA) 99 (MSSA) | 3 (MRSA) 72 (MSSA) |
| Guitguizova et al. (2001) [17] | Urine | Hospital | 36 | 12 | 33 | 100 (MRSA) 100 (MSSA) | 25 (MRSA) 87.5 (MSSA) | 25 (MRSA)b 87.5 (MSSA) |
| Wagelhener et al. (2008) [3] | Urine | Urology unit | 200 | 34 | 17 | 100 (SA) | 81 (SA) | 75 (SA)b |
| Linhares et al. (2013) [14] | Urine | Community | 1128 | 192a | 17a | 97 (SA) | 94 (SA) | 82 (SA)b |
| Jones et al. (2013) [22] | Blood | Hospital | 1036 | 522 | NR | 100 (MRSA) 100 (MSSA) | NR | 29 (MRSA)c 86 (MSSA) |
| Mendes et al. (2014) [21] | Blood | Hospital | 9115 | 3448 | 38 | 100 (MRSA) 100 (MSSA) | NR | 22 (MRSA)c 89 (MSSA) |
-
-
Stamm WE, Norrby SR (2001) Urinary tract infections: disease panorama and challenges. J Infect Dis 183 Suppl 1: doi: https://doi.org/10.1086/318850. [View Article] [PubMed] [Google Scholar]
-
Grude N, Tveten Y, Kristiansen BE (2001) Urinary tract infections in Norway: bacterial etiology and susceptibility. A retrospective study of clinical isolates. Clin Microbiol Infect 7: 543-547. doi: https://doi.org/10.1046/j.1198-743x.2001.00306.x. [View Article] [PubMed] [Google Scholar]
-
Wagenlehner FME, Niemetz AH, Weidner W, Naber KG (2007) Spectrum and antibiotic resistance of uropathogens from hospitalised patients with urinary tract infections: 1994-2005. Int J Antimicrob Agents 31 Suppl 1: doi: https://doi.org/10.1016/j.ijantimicag.2007.07.029. [View Article] [PubMed] [Google Scholar]
-
Baraboutis IG, Tsagalou EP, Lepinski JL, Papakonstantinou I, Papastamopoulos V, et al. (2010) Primary Staphylococcus aureus urinary tract infection: the role of undetected hematogenous seeding of the urinary tract. Eur J Clin Microbiol Infect Dis 29: 1095-1101. doi: https://doi.org/10.1007/s10096-010-0967-2. [View Article] [PubMed] [Google Scholar]
-
Choi S, Lee S, Choi J, Lim SK, Chung J, et al. (2009) The clinical significance of concurrent Staphylococcus aureus bacteriuria in patients with S. aureus bacteremia. J Infect 59: 37-41. doi: https://doi.org/10.1016/j.jinf.2009.05.002. [View Article] [PubMed] [Google Scholar]
-
Chihara S, Popovich KJ, Weinstein RA, Hota B (2010) Staphylococcus aureus bacteriuria as a prognosticator for outcome of Staphylococcus aureus bacteremia: a case-control study. BMC Infect Dis 10: 225. doi: https://doi.org/10.1186/1471-2334-10-225. [View Article] [PubMed] [Google Scholar]
-
Huggan PJ, Murdoch DR, Gallagher K, Chambers ST (2008) Concomitant Staphylococcus aureus bacteriuria is associated with poor clinical outcome in adults with S. aureus bacteraemia. J Hosp Infect 69: 345-349. doi: https://doi.org/10.1016/j.jhin.2008.04.027. [View Article] [PubMed] [Google Scholar]
-
Routh JC, Alt AL, Ashley RA, Kramer SA, Boyce TG (2009) Increasing prevalence and associated risk factors for methicillin resistant Staphylococcus aureus bacteriuria. J Urol 181: 1694-1698. doi: https://doi.org/10.1016/j.juro.2008.11.108. [View Article] [PubMed] [Google Scholar]
-
Al Mohajer M, Musher DM, Minard CG, Darouiche RO (2013) Clinical significance of Staphylococcus aureus bacteriuria at a tertiary care hospital. Scand J Infect Dis 45: 688-695. doi: https://doi.org/10.3109/00365548.2013.803291. [View Article] [PubMed] [Google Scholar]
-
Shurland S, Zhan M, Bradham DD, Roghmann M (2007) Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 28: 273-279. doi: https://doi.org/10.1086/512627. [View Article] [PubMed] [Google Scholar]
-
Muder RR, Brennen C, Rihs JD, Wagener MM, Obman A, et al. (2005) Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin Infect Dis 42: 46-50. doi: https://doi.org/10.1086/498518. [View Article] [PubMed] [Google Scholar]
-
Sheth S, DiNubile MJ (1997) Clinical significance of staphylococcus aureus bacteriuria without concurrent bacteremia. Clin Infect Dis 24: 1268-1269. doi: https://doi.org/10.1093/clinids/24.6.1268. [View Article] [PubMed] [Google Scholar]
-
Brown DFJ, Edwards DI, Hawkey PM, Morrison D, Ridgway GL, et al. (2005) Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J Antimicrob Chemother 56: 1000-1018. doi: https://doi.org/10.1093/jac/dki372. [View Article] [PubMed] [Google Scholar]
-
Linhares I, Raposo T, Rodrigues A, Almeida A (2013) Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: a ten-year surveillance study (2000-2009). BMC Infect Dis 13: 19. doi: https://doi.org/10.1186/1471-2334-13-19. [View Article] [PubMed] [Google Scholar]
-
Sorlozano A, Jimenez-Pacheco A, de Dios Luna Del Castillo, (Juan) , Sampedro A, Martinez-Brocal A, et al. (2014) Evolution of the resistance to antibiotics of bacteria involved in urinary tract infections: a 7-year surveillance study. Am J Infect Control 42: 1033-1038. doi: https://doi.org/10.1016/j.ajic.2014.06.013. [View Article] [PubMed] [Google Scholar]
-
Zhanel GG, Hisanaga TL, Laing NM, DeCorby MR, Nichol KA, et al. (2006) Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents 27: 468-475. doi: https://doi.org/10.1016/j.ijantimicag.2006.02.009. [View Article] [PubMed] [Google Scholar]
-
Guirguitzova B, Chankova D, Zozikov B (2002) [Staphylococci as uropathogens-frequency of isolation in hospitalized patients and sensitivity to antimicrobial agents]. Ann Urol (Paris) 36: 341-347. doi: https://doi.org/10.1016/S0003-4401(02)00124-9. [View Article] [PubMed] [Google Scholar]
-
Schneeberger C, Holleman F, Geerlings SE (2016) Febrile urinary tract infections: pyelonephritis and urosepsis. Curr Opin Infect Dis 29: 80-85. doi: https://doi.org/10.1097/QCO.0000000000000227. [View Article] [PubMed] [Google Scholar]
-
Yasmin M, El Hage H, Obeid R, El Haddad H, Zaarour M, et al. (2015) Epidemiology of bloodstream infections caused by methicillin-resistant Staphylococcus aureus at a tertiary care hospital in New York. Am J Infect Control 44: 41-46. doi: https://doi.org/10.1016/j.ajic.2015.08.005. [View Article] [PubMed] [Google Scholar]
-
Grayson ML, Russo PL, Cruickshank M, Bear JL, Gee CA, et al. (2011) Outcomes from the first 2 years of the Australian National Hand Hygiene Initiative. Med J Aust 195: 615-619. doi: https://doi.org/10.5694/mja11.10747. [View Article] [PubMed] [Google Scholar]
-
Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN (2014) Oritavancin activity against Staphylococcus aureus causing invasive infections in U.S. and European hospitals: a 5-year international surveillance program. Antimicrob Agents Chemother 58: 2921-2924. doi: https://doi.org/10.1128/AAC.02482-13. [View Article] [PubMed] [Google Scholar]
-
Jones RN, Sader HS, Flamm RK (2013) Update of dalbavancin spectrum and potency in the USA: report from the SENTRY Antimicrobial Surveillance Program (2011). Diagn Microbiol Infect Dis 75: 304-307. doi: https://doi.org/10.1016/j.diagmicrobio.2012.11.024. [View Article] [PubMed] [Google Scholar]
-
Weber SG, Gold HS, Hooper DC, Karchmer AW, Carmeli Y (2003) Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis 9: 1415-1422. doi: https://doi.org/10.3201/eid0911.030284. [View Article] [PubMed]
-
Therapeutic Guidelines Limited. Severe sepsis and septic shock: Staphylococcus aureus [Internet]. Melbourne, Australia: Australian Government, Department of Health. July 2017 [Accessed 10 September 2016]. Available from: http://www.tg.org.au/
-
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, et al. (2011) Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52: doi: https://doi.org/10.1093/cid/ciq146. [View Article] [PubMed] [Google Scholar]
-
Felten A, Grandry B, Lagrange PH, Casin I (2002) Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): a disk diffusion method with cefoxitin and moxalactam, the Vitek 2 system, and the MRSA-screen latex agglutination test. J Clin Microbiol 40: 2766-2771. doi: https://doi.org/10.1128/JCM.40.8.2766-2771.2002. [View Article] [PubMed] [Google Scholar]
-
Skov R, Smyth R, Clausen M, Larsen AR, Frimodt-Møller N, et al. (2003) Evaluation of a cefoxitin 30 microg disc on Iso-Sensitest agar for detection of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 52: 204-207. doi: https://doi.org/10.1093/jac/dkg325. [View Article] [PubMed] [Google Scholar]
-

