INTRODUCTION
Inflammatory myofibroblastic tumor (IMT) is considered to be a benign form of lesion comprised of proliferation of myofibroblasts with varying degree of inflammatory infiltrates. It is one of the rare lesions mainly affecting the soft tissue and visceral regions of children and young adults though its occurrence in older population has also been reported in few cases, oldest reported one being 71 year old male [
1].
IMT is also known with other descriptive terms, namely, inflammatory pseudotumor, pseudosarcoma, myxoid hamartoma, plasma cell granuloma and inflammatory myofibrohistiocytic proliferation [
2]. The lesion was initially described in the lung specimen by Bunn in 1939. In 2002, World Health Organization (WHO), it was classified as a soft tissue tumor and named as “inflammatory myofibroblastic tumor” [
3]. The exact etiology of IMT is still unknown but reactive, autoimmune, infectious and trauma has been suggested as contributing factors for its pathogenesis. The most common site for IMT include lung, liver and mesentry and omentum but other rare sites have also reported such as oral cavity, upper gastrointestinal, sinuses and salivary glands [
4]. IMT is mainly considered as a benign lesion with reactive inflammatory process but has potential for recurrence and at few instances has an aggressive course with tendency for malignant transformation and metastases [
1].
IMT of urinary bladder is one of the common sites for the IMTs arising in the genitourinary tract. IMT of urinary bladder has predilection for male and occurs in the 4–5
th decade of life. It usually presents with clinical features such as hematuria, lower abdominal pain, infection with or without fever. The mass usually appears as nodular or frank polypoid lesion measuring in size from 1.5 to 13 cm. From the pathological standpoint, grossly the resected specimen show firm to hard, whorled, or gelatinous cut surface with or without area of ulceration and necrosis [
3,
5].
We here present a unique case of 93-year old female with no prior history of urinary issues or surgical procedure who demonstrated three episodes of hematuria with polypoidal bladder mass. The pathological analysis of all the resected specimen were consistent with the diagnosis of inflammatory myofibroblastic tumor. Our report highlights the rare case of IMT at rare location in uncommon age demonstrating rapid course and exhibiting malignant transformation in short period of time. Considering the rapid transformation of the lesion from benign to malignant stage, the patient has been closely followed-up.
CASE PRESENTATION
A 93-year old female with no prior history of urinary complaints or surgical procedure came to hospital with frequent episodes of a five-day long gross hematuria. The renal ultrasound performed at primary care a day prior to presentation at the hospital revealed mass in the bladder. The urine based laboratory work-up at the hospital revealed red cloudy urine with pH 5.0, negative nitrite and leukocyte esterase tests. The urinary white blood cell (WBC) and red blood cell (RBC) count were 2311 and 1844, respectively. The BUN and creatinine levels were 36.5 mg/dl and 139 mg/dl, respectively. The CT scan of abdomen showed large bladder mass occupying the posterior and right lateral wall of the urinary bladder (Fig. 1).
The cystoscopy procedure revealed a large mass with a stalk on the right posterolateral wall of the bladder near the dome. The tumor appeared to be very hard with areas of necrosis and measuring more than 5.0 cm in the greatest dimension. The transurethral resection procedure was performed and mass was visibly completely resected and sent for pathological examination. Histologically, specimen showed predominantly lesional cells comprised of spindle or stellate shaped cells with mild myxoid stroma and scattered numerous inflammatory cells. No bladder muscle component was identified in the specimen (Fig. 2).
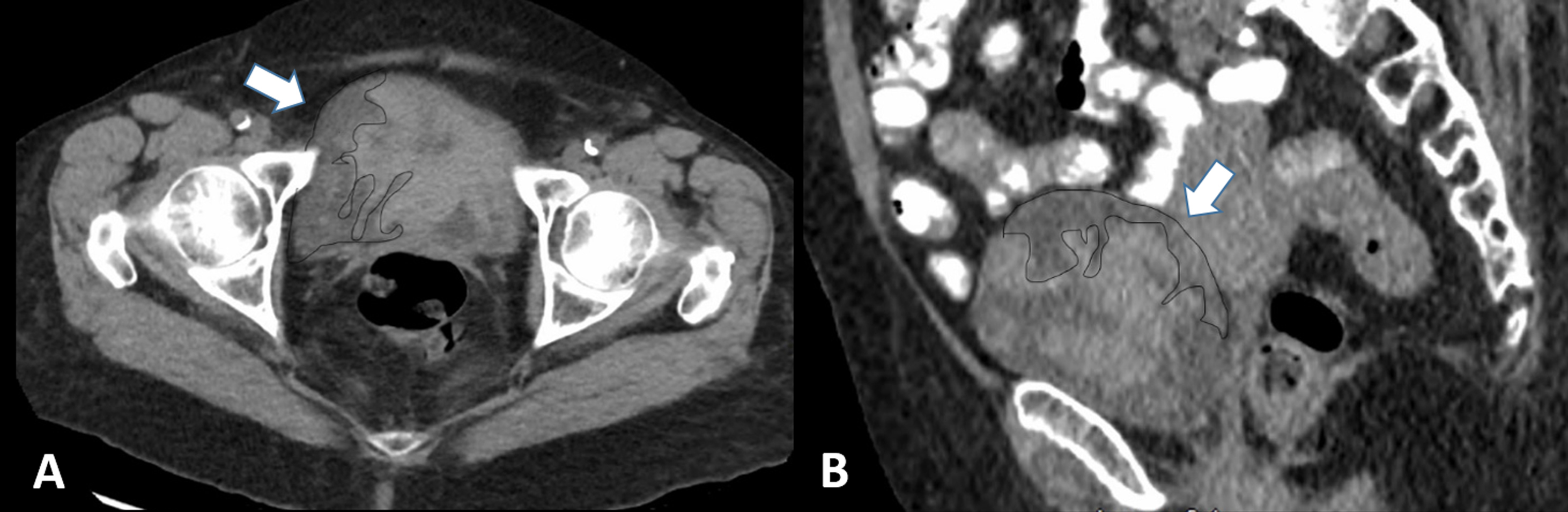
Figure 1. Computer tomography scan images of lower abdomen. A. Axial CT with IMT (arrow and dotted) in the urinary bladder. B. Coronal CT with IMT (arrow and dotted) in the urinary bladder.
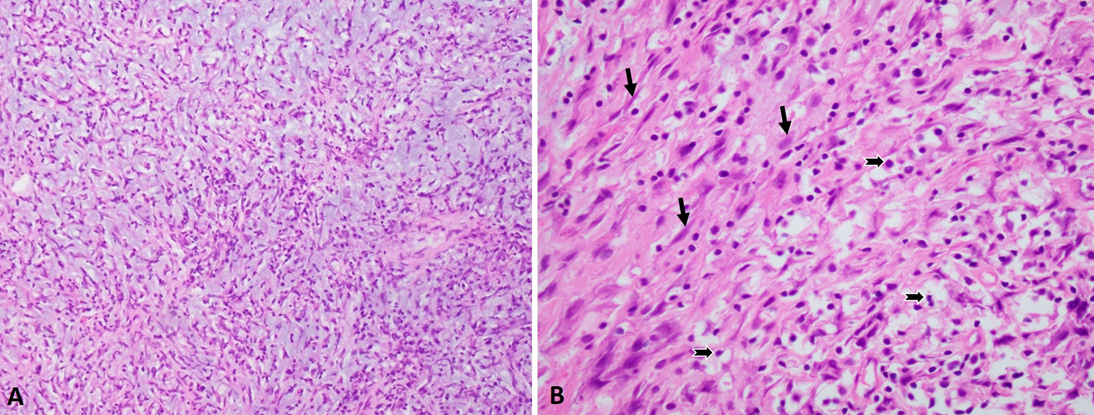
Figure 2. Inflammatory myofibroblastic tumor in the urinary bladder. A. Low-power view. B. High power view showing spindle cells (arrows) with interspersed inflammatory cells (notched arrows).
The IHC studies were performed to reveal diffuse positive reactivity for pankeratin and focally for smooth muscle actin but no reactivity for desmin and p63 confirming the diagnosis for inflammatory myofibroblastic tumor (Fig. 3A-3D). The immunohistochemistry study for anaplastic lymphoma kinase (ALK) expression was negative (Fig. 3E). We also performed FISH studies using break-apart probe to determine ALK gene rearrangement at 2p23 locus. The tumor cell failed to demonstrate 2p23 gene rearrangement as well as any reactivity for uroplakin II (Fig. 4). The lack of expression of p63, uroplakin II and GATA 3 (data not shown as only inflammatory cells showed positive reactivity for GATA 3) ruled out possibility of sarcomatoid urothelial carcinoma.
Six months later, the patient again presented with another episode of hematuria. The cystoscopy revealed bladder mass at the previously resected site and was completely resected. The pathological analysis again confirmed the diagnosis of IMT with histological features similar to those of previous resected tumor. Three months later, the patient again admitted to the hospital with complaints of gross hematuria. She again underwent cystoscopy with re-resection of the necrotic mass occurring at the previous tumor site via transuretheral resection procedure. However, the histopathological analysis of the tumor showed numerous mitotic figures counting up to 25 per 10 high power field with high Ki67 and p53 expression (Fig. 5).
These findings support the ongoing malignant transformation of the tumor. For last two months since her last visit, patient has no complaint and being followed-up very closely.
DISCUSSION
An inflammatory myofibroblastic tumor is a rare benign type of tumor comprised of myofibroblastic spindle cells with variable amount of inflammatory cells [
3]. It is known to occur in young patients usually less than age 40 year and can occur at pulmonary and extrapulmonary sites with a mean age of approximately 10 years. Females are slightly more commonly affected than males. Few reports have highlighted its occurrence at rare site and at older ages [
1,
5]. Some of the most common extrapulmonary sites include orbit, peritoneum, mesentry and head and neck regions [
4]. The signs and symptoms for IMT depend on the site of the tumor. Most of the IMTs are asymptomatic with mild pressure related symptoms, while pulmonary ones demonstrate nonspecific respiratory symptoms, fever, or pain.
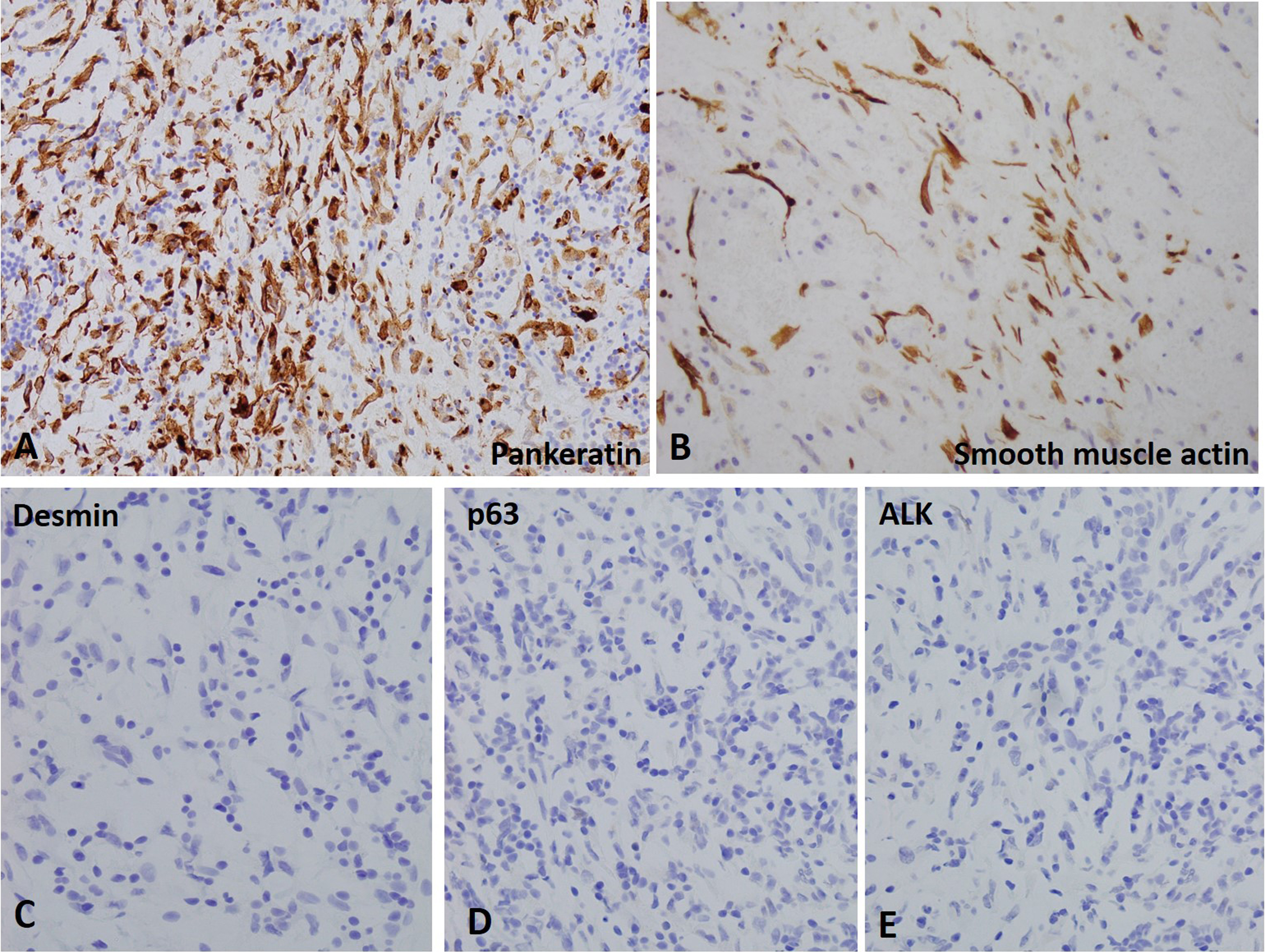
Figure 3. Immunohistochemical stains for inflammatory myofibroblastic tumor in the urinary bladder. A and B. Tumor cells show positive reactivity for pankeratin and smooth muscle actin. C-E. Tumor cells show negative reactivity for desmin, p63 and ALK.
IMT is considered to be very rare at genitourinary tract. The first case of IMT of urinary bladder was reported by Roth in 1980 [
6]. The average age is described as 38.9 years with male predominance [
5]. Other genitourinary sites for IMT include kidney, urethra, ureter, prostate and rete testis [
5]. The common symptoms include hematuria, urinary frequency and suprapubic pain. Grossly, the tumor is usually well circumscribed lesion with myxoid or firm homogenous cut surface. In our patient, the CT scan and cystoscopic examination of the lesion demonstrated a large (> 5 cm) hard mass with necrotic areas arising from the right posterolateral wall of the bladder near the dome (
Fig. 1). The most common pathological presentation of IMT includes myofibroblastic and fibroblastic spindle cells with variable inflammatory infiltrate of lymphocytes, plasma cells, eosinophils and histiocytes. It is classically associated with 3 histologic patterns with IMT displaying all three histological patterns or predominantly one or two. The first is a myxoid pattern with loosely arranged fusiform cells in a myxoid stroma with prominent vasculature. The inflammatory infiltrate predominantly composed of neutrophils and eosinophils with few plasma cells. The second pattern consists of compact spindle cells showing cellular proliferation of spindle cells with arrangement in fascicular or storiform form. The spindle cells are interspersed with mainly plasma cells and lymphocytes. The third pattern is characterized by hypocellular fibrous pattern with elongated spindle cells in the background of dense collagenous stroma with scattered eosinophils, lymphocytes and plasma cells [
5]. In our case, the spindle cells were arranged in short fascicles with a storiform pattern with some areas of hypocellularity with background of myxoid. The spindle cells displayed ovoid to fusiform nuclei with eosinophilic cytoplasm and nuclei with uniform chromatin pattern. Mitotic figures and necrosis were not readily apparent at the initial presentation (
Fig. 2). The immunohistochemical studies for IMT of bladder show focal and or diffuse positive reactivity for pankeratin, Cam 5.2, CK 18, actin, desmin, calponin and smooth muscle actin with lack of S100, CD 34 and myogenin [
7]. The immunohistochemical stains help in diagnosis of IMT from other entities considered as differential diagnosis for IMT and include sarcomatoid carcinoma, rhabdomyosarcoma and leiomyosarcoma. The sarcomatoid carcinoma exhibit epithelial and mesenchymal components with tumor cells composed of spindle cells arranged in fascicular pattern. Especially the differentiation between sarcomoid carcinoma and IMT could be challenging in case of IMT displaying increased mitoses, high cellularity and invasion into muscularis propria. Furthermore, p63 is usually positive in sarcomatoid carcinoma but negative in IMT. Leiomyosarcoma and rhabdomyosarcoma demonstrate diffuse positive reactivity for desmin and myogenin, respectively [
5]. The lesional cells in our case showed negative expression of p63 and desmin but positive staining for pankeratin, smooth muscle myosin (
Fig. 3). Furthermore, the lesional cells lack expression of GATA-3 and uroplakin II thereby ruling out sarcomatoid urothelial carcinoma (
Fig. 4B). The recurrence of IMT at the previously resected site after complete resection indicates a de novo process for subsequent tumors, however; recurrence due to unintentional incomplete resection cannot be ruled out.
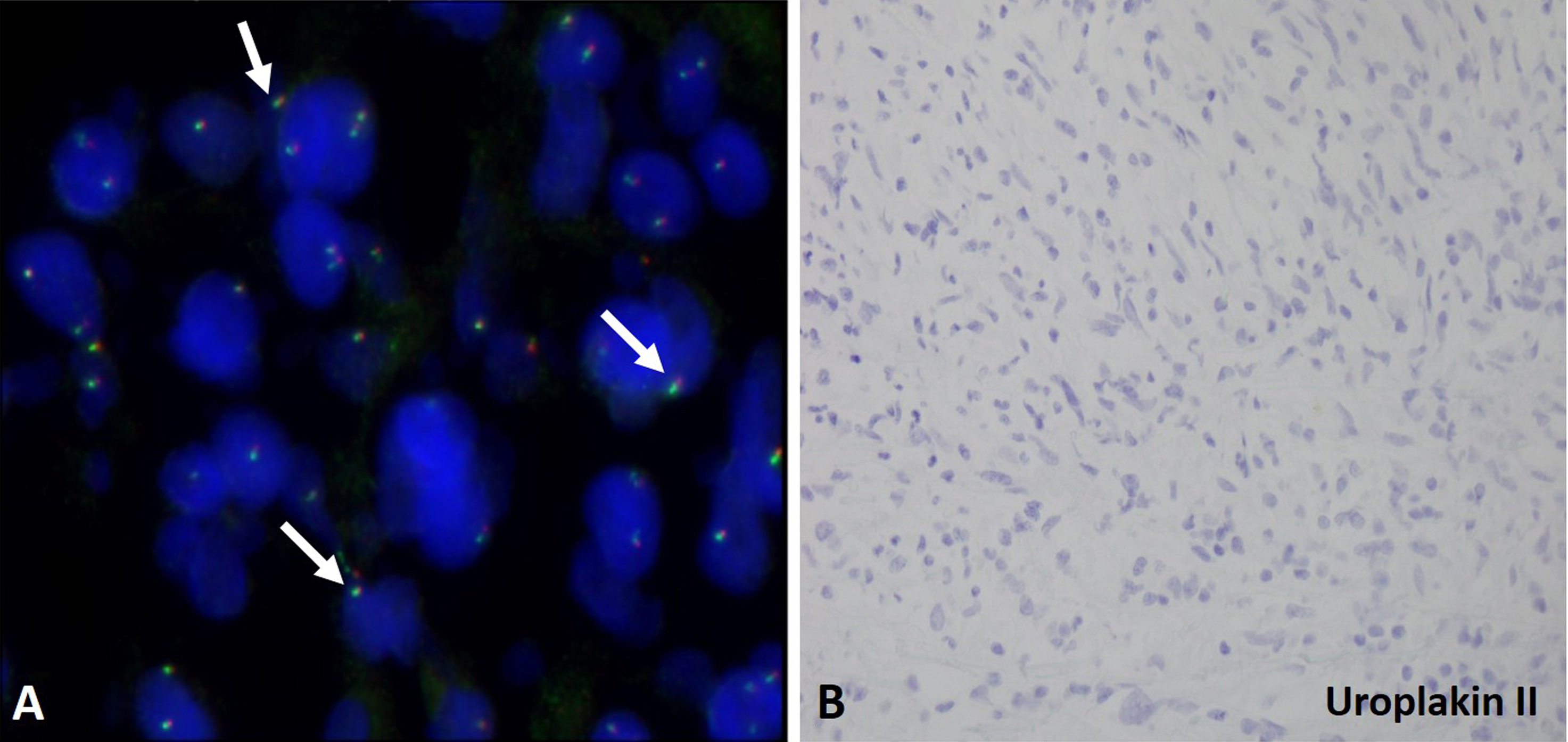
Figure 4. FISH and immunohistochemistry studies for inflammatory myofibroblastic tumor in the urinary bladder. A. The tumor cells failed to show 2p23 ALK gene rearrangement. The arrows indicate the intact juxtaposed green and red signals in the tumor cells. B. Tumor cells showed no reactivity (lack of brown staining cells) for uroplakin II.
Interestingly, the tumor cells lack anaplastic lymphoma kinase (ALK) reactivity as well as ALK gene rearrangement in our case (
Fig. 3E and
Fig. 4A). Most of the IMTs involve clonal rearrangements of ALK gene located on chromosome 2p23 leading to the overexpression of ALK cases and positive cytoplasmic immunostaining for ALK in 50% IMT cases [
8]. In addition, several ALK fusion partners have been identified where ALK fusions arise from fusion of the 3’ end of the ALK gene (exons 20–29) with the 5’ region of a different gene such as TPM3, TPM4, CLTC, RANBP2, CARS, SEC1L1 and ATIC [
7,
8]. The ALK rearrangement by FISH can substantiate IHC findings although in rare case other none-ALK translocations such as TFG-ROS1 and ETV6-NTRK have also been identified [
9]. Usually, ALK reactivity rules out other entities in the differential diagnosis for IMT. It has been suggested that ALK-positive is associated with a more favorable prognosis. One study found that a higher percentage of patients with localized disease were ALK-positive (about 60%) as compared to those without ALK-expression. Taylor
et al. (2019) demonstrated the utility of next generation sequencing especially for ALK negative IMT cases in patients where ROS1 and NTRK3 fusions especially for cases displaying morphological similarity to IgG4 related diseases [
9].
Variable reactivity for p53 and Ki67 has been reported in IMT (Montgomery
et al., 2008). In our case, the expression level for p53 and Ki67 was relatively lower in the TUR specimen collected at the time of initial presentation (
Fig. 4C and
4E). However, the resected specimen collected nine month after the initial presentation during the third visit, there was increased mitotic activity (upto 25 per 10 hpf) was identified in the specimen and the expression level for p53 and Ki67 was relatively higher as compared to initial presentation (
Fig. 4D and
4F). P53 and Ki67 have been identified as markers of malignant transformation of other soft tissue lesions as well as useful tools for discriminating between the stages of the progressive cervical disease [
10]. Although, such association is needed to be validated with additional larger studies, the simple immunohistochemical studies for p53 and Ki67 can be useful to identify the malignant potential of IMT. Though rare, the malignant transformation of IMT is known to cause either local or distant metastasis with poor prognosis [
1]. A close follow via routine urine analysis and cystoscopy is highly recommended in such cases. Since the last visit, our patient has no urinary issues and is being followed-up very closely.
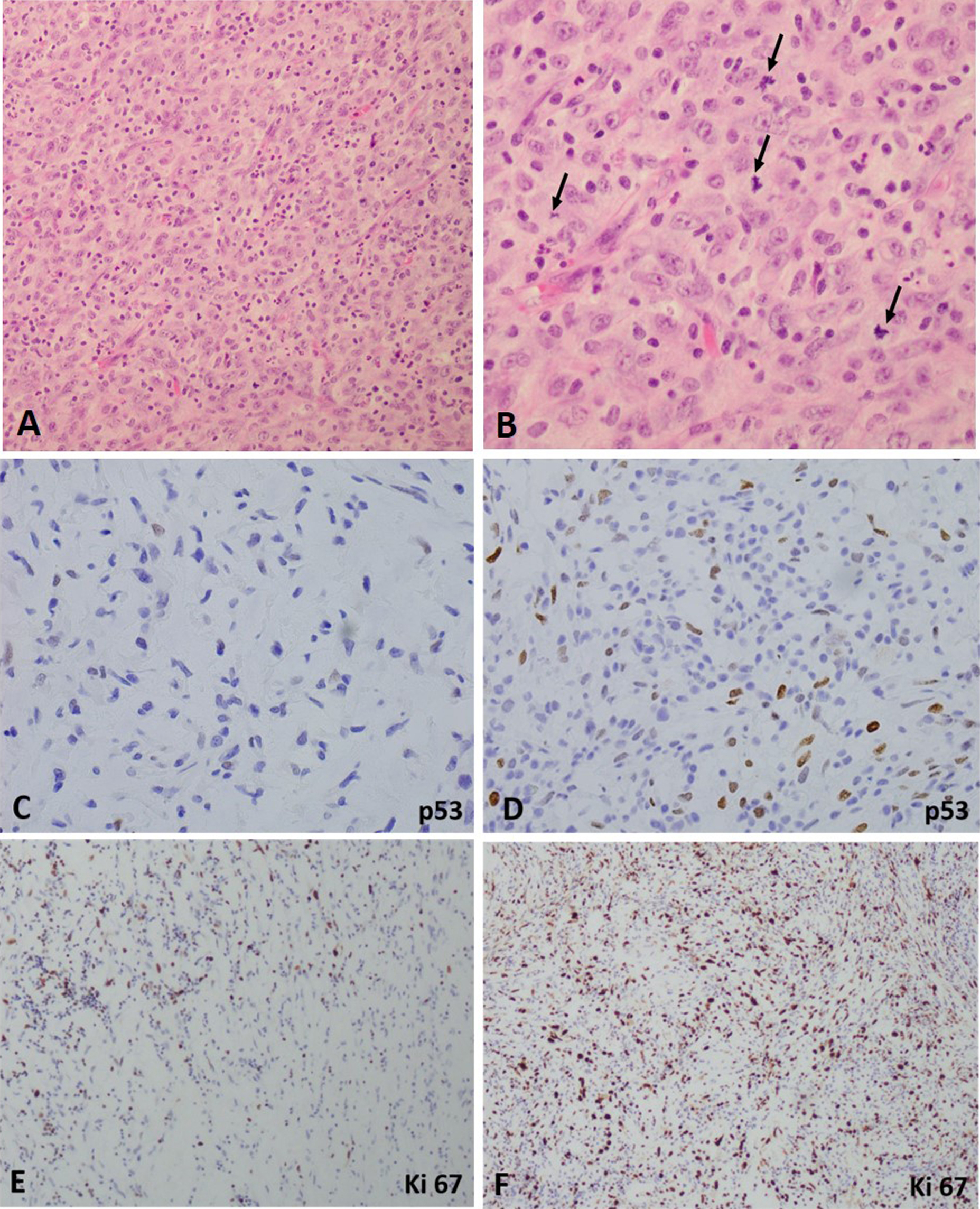
Figure 5. Hemotoxylin and eosin and immunohistochemical stains for malignant transformation of inflammatory myofibroblastic tumor in the urinary bladder. A. Low-power view. B. High power view showing tumor cells undergoing mitosis (arrows). C and E. Tumor cells at the initial presentation show minimal positive reactivity for p53 and Ki67. D and F. Tumor cells from the resected specimen after third recurrence show extensive positive reactivity for p53 and Ki67.
CONCLUSION
Our report presents a rare case of IMT in an older patient with rapid progression to malignant form indicating the un-predictive nature of IMT. In such cases, close follow up is highly recommended.







