Repeat transurethral resection is still an essential tool in treating non-muscle invasive bladder cancer: the Western Australian experience

Objectives: To determine the rate of residual disease and under-staging after primary transurethral resection (TUR) of bladder tumors (TURBT) in tertiary hospitals in Western Australia.
Methods: A retrospective study was performed evaluating all patients with TaHG (stage Ta, high-grade), T1LG (stage T1, low-grade) or T1HG (stage T1, high-grade) bladder cancer on primary TURBT conducted between January 1, 2012 and December 31, 2017 at the four largest metropolitan public hospitals in Western Australia. Only patients who underwent repeat resection within 3 months from initial resection were included. Those with previous history of bladder cancer, incomplete follow-up data and visibly incomplete initial resection were excluded. Baseline patient demographics, macroscopic clearance at initial resection, and disease data at initial and repeat resections were recorded.
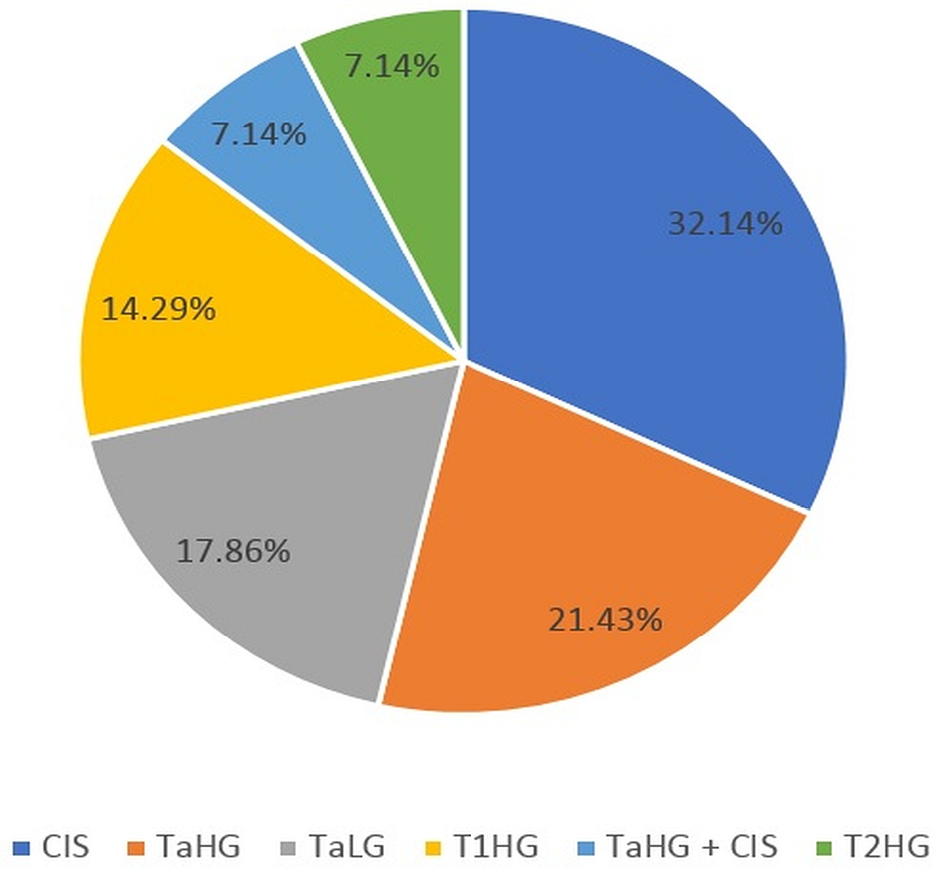
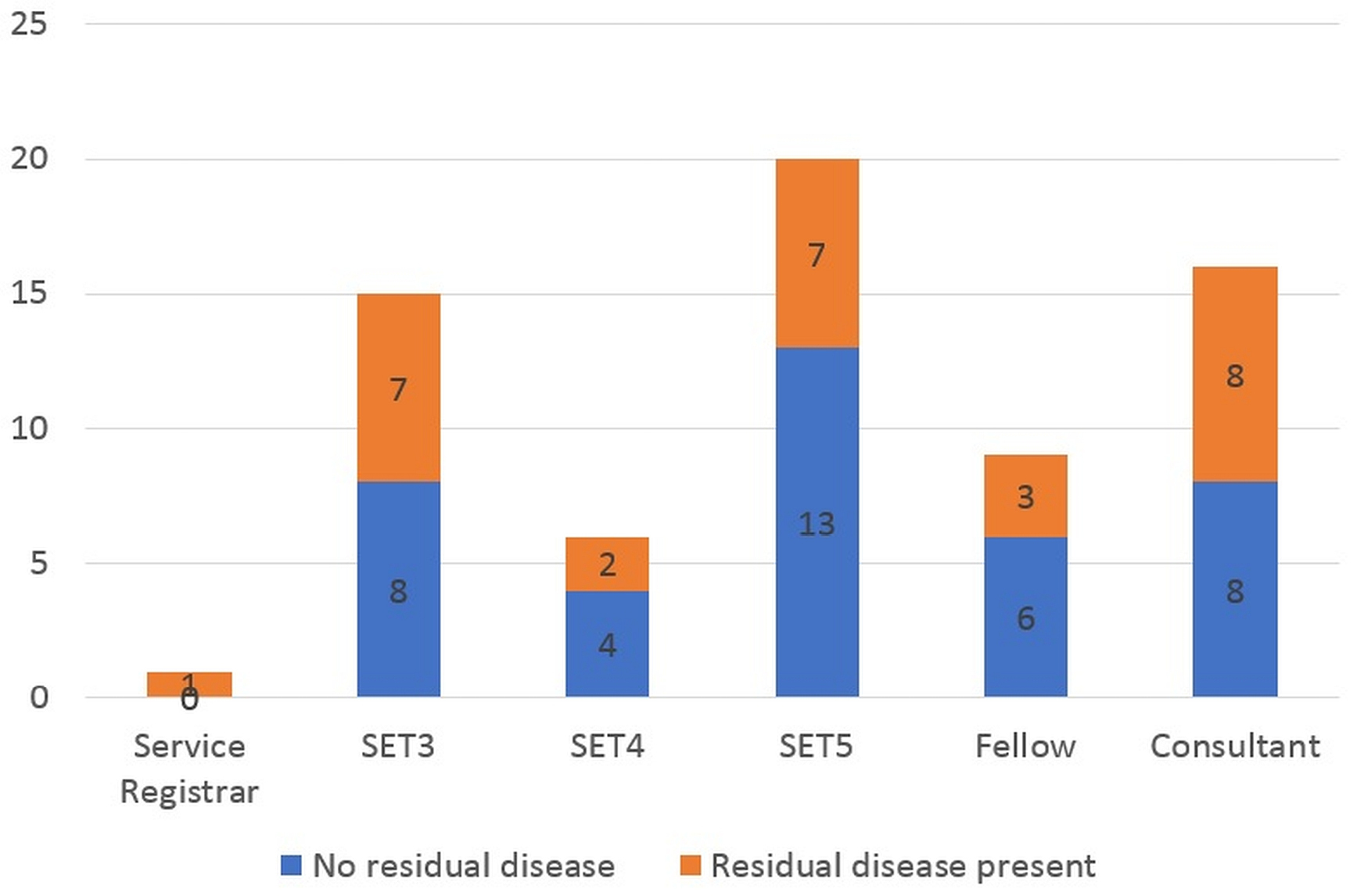
Results: Sixty-seven patients with a median age of 71 years were included in this study. At initial resection, T1HG was the most common disease stage (64.2%) and detrusor muscle was present in 82.1% of initial resections. At repeat resection, 41.8% of cases had residual disease. The rate of upstaging to muscle-invasive bladder cancer was 3.0%. Patients treated by operators with five or less years of formal training did not have a significantly different rate of residual disease from patients treated by operators with more than five years of experience.
Conclusions: Repeat TUR should remain an essential practice due to high rates of residual disease and a small risk of tumor under-staging. The presence of detrusor muscle and macroscopic clearance should not be used as surrogates for adequacy of resection or consideration of avoiding a repeat TUR, even for TaHG disease.
INTRODUCTION
METHODS
RESULTS
DISCUSSION
-
-
Australian Institute of Health and Welfare (2017) Cancer in Australia 2017. Canberra: Australian Institute of Health and Welfare. Cat. No: CAN 100. 204 p. [Last updated September 8, 2017; Cited on January 27, 2019]. Available from: https://www.aihw.gov.au/getmedia/3da1f3c2-30f0-4475-8aed-1f19f8e16d48/20066-cancer-2017.pdf.aspx?inline=true.
-
Ebrahimi H, Amini E, Pishgar F, Moghaddam SS, Nabavizadeh B, et al. (2019) Global, Regional and National Burden of Bladder Cancer, 1990 to 2016: Results from the GBD Study 2016. J Urol 201: 893-901.doi: https://doi.org/https://doi.org/10.1097/JU.0000000000000025. [View Article] [PubMed] [Google Scholar]
-
Cumberbatch MGK, Foerster B, Catto JWF, Kamat AM, Kassouf W, et al. (2018) Repeat Transurethral Resection in Non-muscle-invasive Bladder Cancer: A Systematic Review. Eur Urol 73: 925-933.doi: https://doi.org/https://doi.org/10.1016/j.eururo.2018.02.014. [View Article] [PubMed] [Google Scholar]
-
Minardi D, Milanese G, Parri G, Lacetera V, Muzzonigro G (2016) Non-muscle invasive high grade urothelial carcinoma of the bladder. Which factors can influence understaging at the time of radical cystectomy?. Arch Ital Urol Androl 88: 13-16.doi: https://doi.org/https://doi.org/10.4081/aiua.2016.1.13. [View Article] [PubMed] [Google Scholar]
-
Divrik T, Yildirim U, Eroğlu AS, Zorlu F, Ozen H (2006) Is a second transurethral resection necessary for newly diagnosed pT1 bladder cancer?. J Urol 175: 1258-1261.doi: https://doi.org/https://doi.org/10.1016/S0022-5347(05)00689-0. [View Article] [PubMed] [Google Scholar]
-
Divrik RT, Sahin AF, Yildirim U, Altok M, Zorlu F (2010) Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol 58: 185-190.doi: https://doi.org/https://doi.org/10.1016/j.eururo.2010.03.007. [View Article] [PubMed] [Google Scholar]
-
Ali MH, Ismail IY, Eltobgy A, Gobeish A (2010) Evaluation of second-look transurethral resection in restaging of patients with nonmuscle-invasive bladder cancer. J Endourol 24: 2047-2050.doi: https://doi.org/https://doi.org/10.1089/end.2010.0319. [View Article] [PubMed] [Google Scholar]
-
Herr HW, Donat SM (2008) Quality control in transurethral resection of bladder tumours. BJU Int 102: 1242-1246.doi: https://doi.org/https://doi.org/10.1111/j.1464-410X.2008.07966.x. [View Article] [PubMed] [Google Scholar]
-
Naselli A, Hurle R, Paparella S, Buffi NM, Lughezzani G, et al. (2017) Role of Restaging Transurethral Resection for T1 Non-muscle invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus 4: 558-567.doi: https://doi.org/https://doi.org/10.1016/j.euf.2016.12.011. [View Article] [PubMed] [Google Scholar]
-
Gontero P, Sylvester R, Pisano F, Joniau S, Oderda M, et al. (2015) The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette-Guérin. BJU Int 118: 44-52.doi: https://doi.org/https://doi.org/10.1111/bju.13354. [View Article] [PubMed] [Google Scholar]
-
Sfakianos JP, Kim PH, Hakimi AA, Herr HW (2013) The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette-Guérin. J Urol 191: 341-345.doi: https://doi.org/https://doi.org/10.1016/j.juro.2013.08.022. [View Article] [PubMed] [Google Scholar]
-
Herr HW (2005) Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to bacillus Calmette-Guerin therapy. J Urol 174: 2134-2137.doi: https://doi.org/https://doi.org/10.1097/01.ju.0000181799.81119.fc. [View Article] [PubMed] [Google Scholar]
-

