Case report
An 82-year-old female presented to the emergency department with rigors, lethargy and back-pain. She reported 12 months of worsening lower urinary tract symptoms with irritative voiding, urgency and recurrent pan sensitive Escherichia coli (E. coli) urine infections. Background history included insulin-dependent diabetes mellitus and non-Hodgkin’s lymphoma, though a she was a non-smoker, and had no occupational exposure to carcinogens. On presentation she was septic. Her abdominal examination was unremarkable. Blood tests revealed a white cell count of 5.4 × 109/L, a haemoglobin of 96 g/L and a serum creatinine of 234 µmol/L. Urine dipstick revealed leukocytes without nitrites or red cells. A non-contrast computed tomography (CT) abdomen showed severe bilateral hydroureteronephrosis and marked thickening of the bladder base involving both vesico-ureteric junctions, suggestive of transitional cell carcinoma (Fig. 1). Antibiotics and resuscitation were commenced, a catheter placed and a cystoscopy arranged.

Figure 1. Computed tomography—coronal (A) and axial (B) planes demonstrating bilateral hydroureteronephrosis and a bladder mass at the base measuring 13 mm.
Intra-operatively a 5 cm polypoid mass with the appearance of muscle invasive bladder transitional cell carcinoma, invading both ureteric orifices was found on the trigone. Several smaller, multifocal satellite lesions situated on the lateral wall were identified. The tumor was resected uncovering both ureteric orifices. Retrograde pyelograms showed tortuous ureters with bilateral hydronephrosis and multiple filling defects (Fig. 2). Bilateral ureteric stents were placed. Resected tumor was sent for histopathological assessment and a staging CT of the chest and bone scan revealed no evidence of metastases.
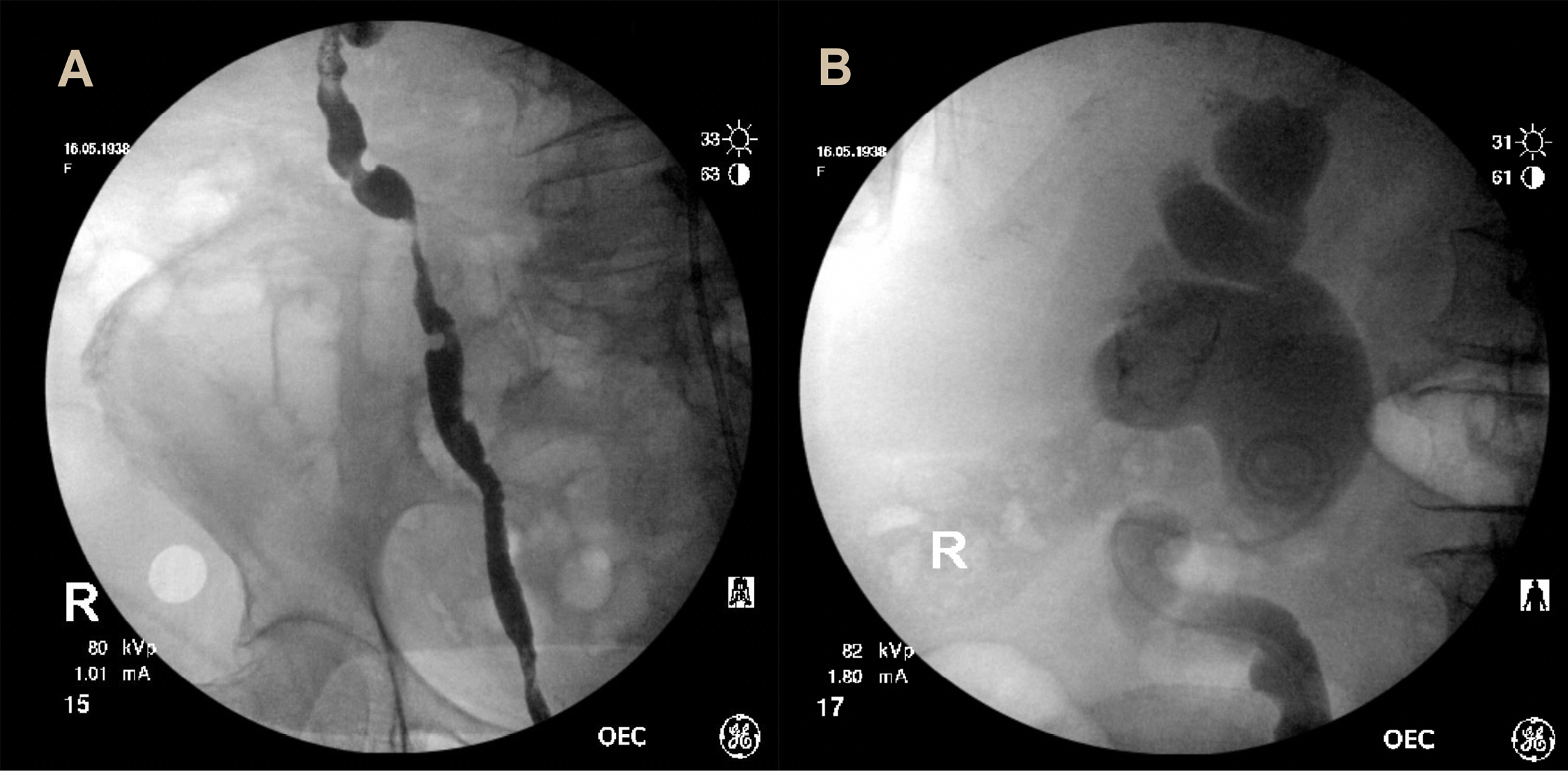
Figure 2. Right retrograde pyelogram revealing multifocal filling defects within the right ureter (A) and right sided hydronephrosis with ureteric stent in situ (B).
The histopathological assessment revealed a mixed inflammatory infiltrate with scattered Michaelis-Gutmann bodies and von Hansemen cells. No evidence of dysplasia or malignancy was seen. The findings were consistent with malakoplakia (Fig. 3). Urine cultures from presentation grew E. coli. Post-operatively the patient improved was discharged with a plan for follow-up cystoscopy and stent removal in 2 months and an extended course of oral amoxicillin-clavulanic acid.
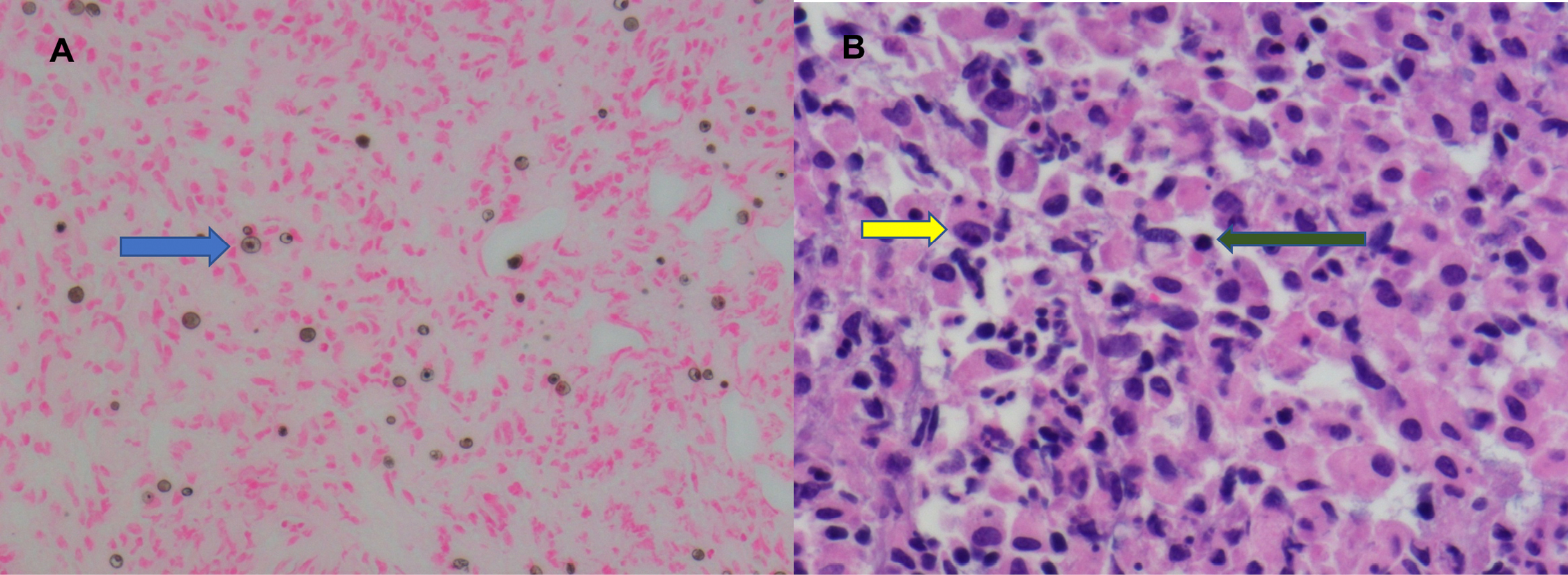
Figure 3. Low (A) and high powered (B) von Kossa calcium stains of the bladder biopsy revealing a mixed inflammatory infiltrate and the presence of von Hansemann histiocytes (blue and yellow arrows) with Michaelis-Gutmann bodies (green arrow).
She represented 2 months later with abdominal pain, an acute kidney injury (serum creatinine of 383 µmol/L) and hyperkalaemia (7.8 mmol/L). Repeat CT imaging revealed persisting bilateral hydroureteronephrosis despite the ureteric stents. A rigid cystoscopy was performed demonstrating an inflamed thick-walled bladder with no residual bladder mass. The ureteric stents were blocked with matrix debris protruding from their lumens. Bilateral retrograde pyelograms showed evidence of persisting hydroureteronephrosis. Eight French ureteric stents were placed. The patient improved and was discharged with plans to undergo a rigid cystoscopy in 3 months’ time.
Malakoplakia is a rare chronic inflammatory condition typically affecting the urinary tract and retroperitoneal tissue [
1]. It is characterized histologically by granulomatous inflammation and by the presence of von Hansemann histiocytes containing intra-cytoplasmic inclusions named Michaelis-Gutmann bodies [
1]. Despite more than a century since first being described, the pathophysiology of the disease remains poorly understood. It is thought to occur secondary to immune system dysregulation and defects in phagocyte function manifesting as granulomatous inflammation [
1]. It is associated with immunosuppression, seen more commonly in patients with lymphoma and diabetes mellitus [
2].
While thought to be a benign condition, this case provides an insight into how malakoplakia can clinically mimic malignancy and cause bilateral ureteric obstruction, resulting in acute renal failure, a finding not previously reported in the literature. Management entails treatment of urinary tract infection if present and surgical resection of the lesions to treat secondary complications and obtain tissue to exclude malignancy [
3]. Where ureteric obstruction is present, renal drainage
via ureteric stents or percutaneous nephrostomy is required. There are no guidelines for the use of antibiotics in preventing recurrence of malakoplakia—both in choice and duration. Studies suggest antibiotics targeting gram negative bacteria (
i.e.,
E. coli) and those that concentrate within macrophages, which may aid in the defective phagocyte function, should be favored [
4,
5]. These include trimethoprim, ciprofloxacin and rifampicin [
4,
5]. Given the adverse effect profile of quinolones, trimethoprim is often preferred. Supplementary vitamin C is advised to reduce the inflammatory response [
6]. For patients presenting with complications secondary to malakoplakia, monitoring of renal function and cystoscopic surveillance is mandated.





